A Trial of a Triple-Drug Treatment for Lymphatic Filariasis
- PMID: 30403937
- PMCID: PMC6194477
- DOI: 10.1056/NEJMoa1706854
A Trial of a Triple-Drug Treatment for Lymphatic Filariasis
Abstract
Background: The World Health Organization has targeted lymphatic filariasis for global elimination by 2020 with a strategy of mass drug administration. This trial tested whether a single dose of a three-drug regimen of ivermectin plus diethylcarbamazine plus albendazole results in a greater sustained clearance of microfilariae than a single dose of a two-drug regimen of diethylcarbamazine plus albendazole and is noninferior to the two-drug regimen administered once a year for 3 years.
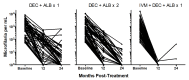
Methods: In a randomized, controlled trial involving adults from Papua New Guinea with Wuchereria bancrofti microfilaremia, we assigned 182 participants to receive a single dose of the three-drug regimen (60 participants), a single dose of the two-drug regimen (61 participants), or the two-drug regimen once a year for 3 years (61 participants). Clearance of microfilariae from the blood was measured at 12, 24, and 36 months after trial initiation.
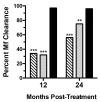
Results: The three-drug regimen cleared microfilaremia in 55 of 57 participants (96%) at 12 months, in 52 of 54 participants (96%) at 24 months, and in 55 of 57 participants (96%) at 36 months. A single dose of the two-drug regimen cleared microfilaremia in 18 of 56 participants (32%) at 12 months, in 31 of 55 participants (56%) at 24 months, and in 43 of 52 participants (83%) at 36 months (P=0.02 for the three-drug regimen vs. a single dose of the two-drug regimen at 36 months). The two-drug regimen administered once a year for 3 years cleared microfilaremia in 20 of 59 participants (34%) at 12 months, in 42 of 56 participants (75%) at 24 months, and in 51 of 52 participants (98%) at 36 months (P=0.004 for noninferiority of the three-drug regimen vs. the two-drug regimen administered once a year for 3 years at 36 months). Moderate adverse events were more common in the group that received the three-drug regimen than in the combined two-drug-regimen groups (27% vs. 5%, P<0.001). There were no serious adverse events.
Conclusions: The three-drug regimen induced clearance of microfilariae from the blood for 3 years in almost all participants who received the treatment and was superior to the two-drug regimen administered once and noninferior to the two-drug regimen administered once a year for 3 years. (Funded by the Bill and Melinda Gates Foundation; ClinicalTrials.gov number, NCT01975441 .).
Figures

Comment in
-
Advancing toward the Elimination of Lymphatic Filariasis.N Engl J Med. 2018 Nov 8;379(19):1871-1872. doi: 10.1056/NEJMe1811455. N Engl J Med. 2018. PMID: 30403953 No abstract available.
References
-
- Taylor MJ, Hoerauf A, Bockarie M. Lymphatic filariasis and onchocerciasis. Lancet 2010;376:1175-85. - PubMed
-
- King J, Biswas G. Global programme to eliminate lymphatic filariasis: progress report, 2015. WHO Weekly Epidemiological Record 2016;91 441–60.
-
- Bockarie MJ, Tisch DJ, Kastens W, et al. Mass treatment to eliminate filariasis in Papua New Guinea. NEJM 2002;347:1841-8. - PubMed
-
- Farid HA, Hammad RE, Hassan MM, Ramzy RM, El Setouhy M, Weil GJ. Effects of combined diethylcarbamazine and albendazole treatment of bancroftian filariasis on parasite uptake and development in Culex pipiens L. Am J Trop Med Hyg 2005;73:108-14. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
