A Screen Using iPSC-Derived Hepatocytes Reveals NAD+ as a Potential Treatment for mtDNA Depletion Syndrome
- PMID: 30404003
- PMCID: PMC6289059
- DOI: 10.1016/j.celrep.2018.10.036
A Screen Using iPSC-Derived Hepatocytes Reveals NAD+ as a Potential Treatment for mtDNA Depletion Syndrome
Abstract
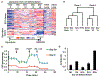
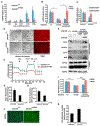
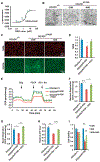
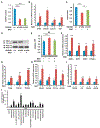
Patients with mtDNA depletion syndrome 3 (MTDPS3) often die as children from liver failure caused by severe reduction in mtDNA content. The identification of treatments has been impeded by an inability to culture and manipulate MTDPS3 primary hepatocytes. Here we generated DGUOK-deficient hepatocyte-like cells using induced pluripotent stem cells (iPSCs) and used them to identify drugs that could improve mitochondrial ATP production and mitochondrial function. Nicotinamide adenine dinucleotide (NAD) was found to improve mitochondrial function in DGUOK-deficient hepatocyte-like cells by activating the peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1α). NAD treatment also improved ATP production in MTDPS3-null rats and in hepatocyte-like cells that were deficient in ribonucleoside-diphosphate reductase subunit M2B (RRM2B), suggesting that it could be broadly effective. Our studies reveal that DGUOK-deficient iPSC-derived hepatocytes recapitulate the pathophysiology of MTDPS3 in culture and can be used to identify therapeutics for mtDNA depletion syndromes.
Keywords: drug screen; iPSC-derived hepatocytes; inborn errors in hepatic metabolism; liver disease; mitochondrial disease.
Copyright © 2018 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
DECLARATION OF INTERESTS
The authors declare no competing interests.
Figures



Similar articles
-
A Combined Model of Human iPSC-Derived Liver Organoids and Hepatocytes Reveals Ferroptosis in DGUOK Mutant mtDNA Depletion Syndrome.Adv Sci (Weinh). 2021 Mar 8;8(10):2004680. doi: 10.1002/advs.202004680. eCollection 2021 May. Adv Sci (Weinh). 2021. PMID: 34026460 Free PMC article.
-
The nucleotide prodrug CERC-913 improves mtDNA content in primary hepatocytes from DGUOK-deficient rats.J Inherit Metab Dis. 2021 Mar;44(2):492-501. doi: 10.1002/jimd.12354. Epub 2021 Jan 15. J Inherit Metab Dis. 2021. PMID: 33368311
-
Comparing the mitochondrial signatures in ESCs and iPSCs and their neural derivations.Cell Cycle. 2022 Oct;21(20):2206-2221. doi: 10.1080/15384101.2022.2092185. Epub 2022 Jul 10. Cell Cycle. 2022. PMID: 35815665 Free PMC article.
-
Role of PGC-1α in the Mitochondrial NAD+ Pool in Metabolic Diseases.Int J Mol Sci. 2021 Apr 27;22(9):4558. doi: 10.3390/ijms22094558. Int J Mol Sci. 2021. PMID: 33925372 Free PMC article. Review.
-
PPARγ-Coactivator-1α, Nicotinamide Adenine Dinucleotide and Renal Stress Resistance.Nephron. 2017;137(4):253-255. doi: 10.1159/000471895. Epub 2017 Jun 8. Nephron. 2017. PMID: 28591759 Free PMC article. Review.
Cited by
-
A Combined Model of Human iPSC-Derived Liver Organoids and Hepatocytes Reveals Ferroptosis in DGUOK Mutant mtDNA Depletion Syndrome.Adv Sci (Weinh). 2021 Mar 8;8(10):2004680. doi: 10.1002/advs.202004680. eCollection 2021 May. Adv Sci (Weinh). 2021. PMID: 34026460 Free PMC article.
-
MSCs vs. iPSCs: Potential in therapeutic applications.Front Cell Dev Biol. 2022 Nov 2;10:1005926. doi: 10.3389/fcell.2022.1005926. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36407112 Free PMC article. Review.
-
The Application of Induced Pluripotent Stem Cells Against Liver Diseases: An Update and a Review.Front Med (Lausanne). 2021 Jul 1;8:644594. doi: 10.3389/fmed.2021.644594. eCollection 2021. Front Med (Lausanne). 2021. PMID: 34277651 Free PMC article. Review.
-
From a Single Cell to a Whole Human Liver: Disease Modeling and Transplantation.Semin Liver Dis. 2022 Nov;42(4):413-422. doi: 10.1055/a-1934-5404. Epub 2022 Aug 31. Semin Liver Dis. 2022. PMID: 36044927 Free PMC article.
-
Advancements in Disease Modeling and Drug Discovery Using iPSC-Derived Hepatocyte-like Cells.Genes (Basel). 2022 Mar 24;13(4):573. doi: 10.3390/genes13040573. Genes (Basel). 2022. PMID: 35456379 Free PMC article. Review.
References
-
- Almannai M, El-Hattab AW, and Scaglia F (2018). Mitochondrial DNA replication: clinical syndromes. Essays Biochem 62, 297–308. - PubMed
-
- Bennett B, Helbling D, Meng H, Jarzembowski J, Geurts AM, Friederich MW, Van Hove JLK, Lawlor MW, and Dimmock DP (2016). Potentially diagnostic electron paramagnetic resonance spectra elucidate the underlying mechanism of mitochondrial dysfunction in the deoxyguanosine kinase deficient rat model of a genetic mitochondrial DNA depletion syndrome. Free Radic. Biol. Med 92, 141–151. - PMC - PubMed
-
- Berger M, and Gastpar M (1996). Trimipramine: a challenge to current concepts on antidepressives. Eur. Arch. Psychiatry Clin. Neurosci 246, 235–239. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

