Hemodynamics in Cardiac Development
- PMID: 30404214
- PMCID: PMC6306789
- DOI: 10.3390/jcdd5040054
Hemodynamics in Cardiac Development
Abstract
The beating heart is subject to intrinsic mechanical factors, exerted by contraction of the myocardium (stretch and strain) and fluid forces of the enclosed blood (wall shear stress). The earliest contractions of the heart occur already in the 10-somite stage in the tubular as yet unsegmented heart. With development, the looping heart becomes asymmetric providing varying diameters and curvatures resulting in unequal flow profiles. These flow profiles exert various wall shear stresses and as a consequence different expression patterns of shear responsive genes. In this paper we investigate the morphological alterations of the heart after changing the blood flow by ligation of the right vitelline vein in a model chicken embryo and analyze the extended expression in the endocardial cushions of the shear responsive gene Tgfbeta receptor III. A major phenomenon is the diminished endocardial-mesenchymal transition resulting in hypoplastic (even absence of) atrioventricular and outflow tract endocardial cushions, which might be lethal in early phases. The surviving embryos exhibit several cardiac malformations including ventricular septal defects and malformed semilunar valves related to abnormal development of the aortopulmonary septal complex and the enclosed neural crest cells. We discuss the results in the light of the interactions between several shear stress responsive signaling pathways including an extended review of the involved Vegf, Notch, Pdgf, Klf2, eNos, Endothelin and Tgfβ/Bmp/Smad networks.
Keywords: Klf2; TGF beta; cardiogenesis; endocardial cushions; growth factors; hemodynamics; neural crest; outflow tract septum; semilunar valve; shear stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
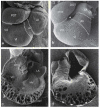

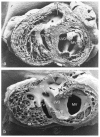

Similar articles
-
Malformations of the semilunar valves produced in chick embryos by mechanical interference with cardiogenesis. An experimental approach to the role of hemodynamics in valvular development.Anat Embryol (Berl). 1983;168(1):59-71. doi: 10.1007/BF00305399. Anat Embryol (Berl). 1983. PMID: 6650857
-
Muscleblind-like 1 is required for normal heart valve development in vivo.BMC Dev Biol. 2015 Oct 15;15:36. doi: 10.1186/s12861-015-0087-4. BMC Dev Biol. 2015. PMID: 26472242 Free PMC article.
-
The role of shear stress on ET-1, KLF2, and NOS-3 expression in the developing cardiovascular system of chicken embryos in a venous ligation model.Physiology (Bethesda). 2007 Dec;22:380-9. doi: 10.1152/physiol.00023.2007. Physiology (Bethesda). 2007. PMID: 18073411 Review.
-
The distribution and characterization of HNK-1 antigens in the developing avian heart.Anat Embryol (Berl). 1993 Sep;188(3):307-16. doi: 10.1007/BF00188221. Anat Embryol (Berl). 1993. PMID: 7504418
-
Control of endocardial cushion and cardiac valve maturation by BMP signaling pathways.Mol Genet Metab. 2003 Sep-Oct;80(1-2):27-35. doi: 10.1016/j.ymgme.2003.07.004. Mol Genet Metab. 2003. PMID: 14567955 Review.
Cited by
-
Computational simulations of the 4D micro-circulatory network in zebrafish tail amputation and regeneration.J R Soc Interface. 2022 Feb;19(187):20210898. doi: 10.1098/rsif.2021.0898. Epub 2022 Feb 16. J R Soc Interface. 2022. PMID: 35167770 Free PMC article.
-
The Development of the Ascending Aortic Wall in Tricuspid and Bicuspid Aortic Valve: A Process from Maturation to Degeneration.J Clin Med. 2020 Mar 26;9(4):908. doi: 10.3390/jcm9040908. J Clin Med. 2020. PMID: 32225051 Free PMC article.
-
New Concepts in the Development and Malformation of the Arterial Valves.J Cardiovasc Dev Dis. 2020 Sep 24;7(4):38. doi: 10.3390/jcdd7040038. J Cardiovasc Dev Dis. 2020. PMID: 32987700 Free PMC article. Review.
-
Mechanosensitive Pathways in Heart Development: Findings from Chick Embryo Studies.J Cardiovasc Dev Dis. 2021 Mar 26;8(4):32. doi: 10.3390/jcdd8040032. J Cardiovasc Dev Dis. 2021. PMID: 33810288 Free PMC article. Review.
-
Engineering Heart Morphogenesis.Trends Biotechnol. 2020 Aug;38(8):835-845. doi: 10.1016/j.tibtech.2020.01.006. Epub 2020 Mar 5. Trends Biotechnol. 2020. PMID: 32673587 Free PMC article. Review.
References
-
- Groenendijk B.C., Hierck B.P., Vrolijk J., Baiker M., Pourquie M.J., Gittenberger-de Groot A.C., Poelmann R.E. Changes in shear stress-related gene expression after experimentally altered venous return in the chicken embryo. Circ. Res. 2005;96:1291–1298. doi: 10.1161/01.RES.0000171901.40952.0d. - DOI - PubMed
LinkOut - more resources
Full Text Sources

