Benzylisoquinoline Alkaloids Biosynthesis in Sacred Lotus
- PMID: 30404216
- PMCID: PMC6278464
- DOI: 10.3390/molecules23112899
Benzylisoquinoline Alkaloids Biosynthesis in Sacred Lotus
Abstract
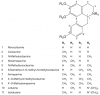
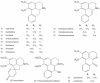
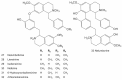
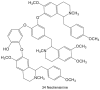
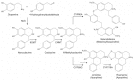
Sacred lotus (Nelumbo nucifera Gaertn.) is an ancient aquatic plant used throughout Asia for its nutritional and medicinal properties. Benzylisoquinoline alkaloids (BIAs), mostly within the aporphine and bisbenzylisoquinoline structural categories, are among the main bioactive constituents in the plant. The alkaloids of sacred lotus exhibit promising anti-cancer, anti-arrhythmic, anti-HIV, and anti-malarial properties. Despite their pharmacological significance, BIA metabolism in this non-model plant has not been extensively investigated. In this review, we examine the diversity of BIAs in sacred lotus, with an emphasis on the distinctive stereochemistry of alkaloids found in this species. Additionally, we discuss our current understanding of the biosynthetic genes and enzymes involved in the formation of 1-benzylisoquinoline, aporphine, and bisbenzylisoquinoline alkaloids in the plant. We conclude that a comprehensive functional characterization of alkaloid biosynthetic enzymes using both in vitro and in vivo methods is required to advance our limited knowledge of BIA metabolism in the sacred lotus.
Keywords: Nelumbo nucifera; benzylisoquinoline alkaloids; cytochrome P450 monooxygenase; medicinal properties; methyltransferase; norcoclaurine synthase; sacred lotus; stereochemistry.
Conflict of interest statement
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures
Similar articles
-
Elucidation of the (R)-enantiospecific benzylisoquinoline alkaloid biosynthetic pathways in sacred lotus (Nelumbo nucifera).Sci Rep. 2023 Feb 20;13(1):2955. doi: 10.1038/s41598-023-29415-0. Sci Rep. 2023. PMID: 36805479 Free PMC article.
-
Isolation and characterization of two O-methyltransferases involved in benzylisoquinoline alkaloid biosynthesis in sacred lotus (Nelumbo nucifera).J Biol Chem. 2020 Feb 7;295(6):1598-1612. doi: 10.1074/jbc.RA119.011547. Epub 2019 Dec 30. J Biol Chem. 2020. PMID: 31914404 Free PMC article.
-
Pathway elucidation and microbial synthesis of proaporphine and bis-benzylisoquinoline alkaloids from sacred lotus (Nelumbo nucifera).Metab Eng. 2023 May;77:162-173. doi: 10.1016/j.ymben.2023.03.010. Epub 2023 Mar 31. Metab Eng. 2023. PMID: 37004909
-
Recent advances on bioactive compounds, biosynthesis mechanism, and physiological functions of Nelumbo nucifera.Food Chem. 2023 Jun 30;412:135581. doi: 10.1016/j.foodchem.2023.135581. Epub 2023 Jan 27. Food Chem. 2023. PMID: 36731239 Review.
-
An updated review on pharmacological properties of neferine-A bisbenzylisoquinoline alkaloid from Nelumbo nucifera.J Food Biochem. 2021 Dec;45(12):e13986. doi: 10.1111/jfbc.13986. Epub 2021 Nov 14. J Food Biochem. 2021. PMID: 34779018 Review.
Cited by
-
Elucidation of the (R)-enantiospecific benzylisoquinoline alkaloid biosynthetic pathways in sacred lotus (Nelumbo nucifera).Sci Rep. 2023 Feb 20;13(1):2955. doi: 10.1038/s41598-023-29415-0. Sci Rep. 2023. PMID: 36805479 Free PMC article.
-
Molecular Origins of Functional Diversity in Benzylisoquinoline Alkaloid Methyltransferases.Front Plant Sci. 2019 Aug 30;10:1058. doi: 10.3389/fpls.2019.01058. eCollection 2019. Front Plant Sci. 2019. PMID: 31543888 Free PMC article. Review.
-
Exploring Chemical Composition, Antioxidant, Enzyme Inhibitory and Cytotoxic Properties of Glaucium acutidentatum Hausskn. & Bornm. from Turkey Flora: A Novel Source of Bioactive Agents to Design Functional Applications.Antioxidants (Basel). 2024 May 25;13(6):643. doi: 10.3390/antiox13060643. Antioxidants (Basel). 2024. PMID: 38929082 Free PMC article.
-
Interplay of transcription factors orchestrating the biosynthesis of plant alkaloids.3 Biotech. 2022 Oct;12(10):250. doi: 10.1007/s13205-022-03316-x. Epub 2022 Aug 29. 3 Biotech. 2022. PMID: 36051988 Free PMC article. Review.
-
The CYP80A and CYP80G Are Involved in the Biosynthesis of Benzylisoquinoline Alkaloids in the Sacred Lotus (Nelumbo nucifera).Int J Mol Sci. 2024 Jan 5;25(2):702. doi: 10.3390/ijms25020702. Int J Mol Sci. 2024. PMID: 38255776 Free PMC article.
References
-
- Xue J., Dong W., Cheng T., Zhou S. Nelumbonaceae: Systematic position and species diversification revealed by the complete chloroplast genome. J. Syst. Evol. 2012;50:477–487. doi: 10.1111/j.1759-6831.2012.00224.x. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

