Cytostretch, an Organ-on-Chip Platform
- PMID: 30404293
- PMCID: PMC6189941
- DOI: 10.3390/mi7070120
Cytostretch, an Organ-on-Chip Platform
Abstract
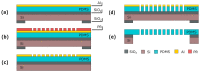
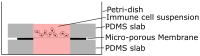
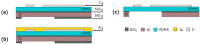
Organ-on-Chips (OOCs) are micro-fabricated devices which are used to culture cells in order to mimic functional units of human organs. The devices are designed to simulate the physiological environment of tissues in vivo. Cells in some types of OOCs can be stimulated in situ by electrical and/or mechanical actuators. These actuations can mimic physiological conditions in real tissue and may include fluid or air flow, or cyclic stretch and strain as they occur in the lung and heart. These conditions similarly affect cultured cells and may influence their ability to respond appropriately to physiological or pathological stimuli. To date, most focus has been on devices specifically designed to culture just one functional unit of a specific organ: lung alveoli, kidney nephrons or blood vessels, for example. In contrast, the modular Cytostretch membrane platform described here allows OOCs to be customized to different OOC applications. The platform utilizes silicon-based micro-fabrication techniques that allow low-cost, high-volume manufacturing. We describe the platform concept and its modules developed to date. Membrane variants include membranes with (i) through-membrane pores that allow biological signaling molecules to pass between two different tissue compartments; (ii) a stretchable micro-electrode array for electrical monitoring and stimulation; (iii) micro-patterning to promote cell alignment; and (iv) strain gauges to measure changes in substrate stress. This paper presents the fabrication and the proof of functionality for each module of the Cytostretch membrane. The assessment of each additional module demonstrate that a wide range of OOCs can be achieved.
Keywords: Cytostretch; customizable; micro-electrode array; micro-grooves; modular; organ-on-chip; platform; stem cells; strain gauges; through-membrane pores.
Conflict of interest statement
The authors declare no conflict of interest.
Figures












References
-
- Gassmann O., Reepmeyer G., von Zedtwitz M. Leading Pharmaceutical Innovation. Springer Science & Business Media; Berlin, Germany: 2008. Trends and drivers for growth in the pharmaceutical industry.
-
- Beißner N., Lorenz T., Reichl S. Microsystems for Pharmatechnology. Springer International Publishing; Gewerbestrasse, Switzerland: 2016. Organ on chip; pp. 299–339.
-
- Jo B.H., van Lerberghe L.M., Motsegood K.M., Beebe D.J. Three-dimensional micro-channel fabrication in polydimethylsiloxane (PDMS) elastomer. J. Microelectromech. Syst. 2000;9:76–81. doi: 10.1109/84.825780. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

