A Y-Shaped Microfluidic Device to Study the Combined Effect of Wall Shear Stress and ATP Signals on Intracellular Calcium Dynamics in Vascular Endothelial Cells
- PMID: 30404384
- PMCID: PMC6190056
- DOI: 10.3390/mi7110213
A Y-Shaped Microfluidic Device to Study the Combined Effect of Wall Shear Stress and ATP Signals on Intracellular Calcium Dynamics in Vascular Endothelial Cells
Abstract
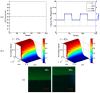
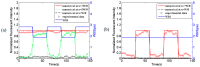
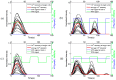
The intracellular calcium dynamics in vascular endothelial cells (VECs) in response to wall shear stress (WSS) and/or adenosine triphosphate (ATP) have been commonly regarded as an important factor in regulating VEC function and behavior including proliferation, migration and apoptosis. However, the effects of time-varying ATP signals have been usually neglected in the past investigations in the field of VEC mechanobiology. In order to investigate the combined effects of WSS and dynamic ATP signals on the intracellular calcium dynamic in VECs, a Y-shaped microfluidic device, which can provide the cultured cells on the bottom of its mixing micro-channel with stimuli of WSS signal alone and different combinations of WSS and ATP signals in one single micro-channel, is proposed. Both numerical simulation and experimental studies verify the feasibility of its application. Cellular experimental results also suggest that a combination of WSS and ATP signals rather than a WSS signal alone might play a more significant role in VEC Ca2+ signal transduction induced by blood flow.
Keywords: Y-shaped microfluidic device; adenosine triphosphate (ATP) signal; calcium dynamics; combined effect; vascular endothelial cells; wall shear stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
A microfluidic device with spatiotemporal wall shear stress and ATP signals to investigate the intracellular calcium dynamics in vascular endothelial cells.Biomech Model Mechanobiol. 2019 Feb;18(1):189-202. doi: 10.1007/s10237-018-1076-x. Epub 2018 Sep 6. Biomech Model Mechanobiol. 2019. PMID: 30187350
-
Modeling of Endothelial Calcium Responses within a Microfluidic Generator of Spatio-Temporal ATP and Shear Stress Signals.Micromachines (Basel). 2021 Feb 7;12(2):161. doi: 10.3390/mi12020161. Micromachines (Basel). 2021. PMID: 33562260 Free PMC article.
-
Modeling of TRPV₄-C₁ -mediated calcium signaling in vascular endothelial cells induced by fluid shear stress and ATP.Biomech Model Mechanobiol. 2015 Oct;14(5):979-93. doi: 10.1007/s10237-015-0647-3. Epub 2015 Jan 11. Biomech Model Mechanobiol. 2015. PMID: 25577546
-
Fluid Shear Stress Sensing by the Endothelial Layer.Front Physiol. 2020 Jul 24;11:861. doi: 10.3389/fphys.2020.00861. eCollection 2020. Front Physiol. 2020. PMID: 32848833 Free PMC article. Review.
-
Flow-induced ATP release in patient-specific arterial geometries--a comparative study of computational models.Int J Numer Method Biomed Eng. 2013 Oct;29(10):1038-56. doi: 10.1002/cnm.2581. Epub 2013 Jul 25. Int J Numer Method Biomed Eng. 2013. PMID: 23894050 Review.
Cited by
-
Comprehensive analysis of lipid nanoparticle formulation and preparation for RNA delivery.Int J Pharm X. 2024 Sep 10;8:100283. doi: 10.1016/j.ijpx.2024.100283. eCollection 2024 Dec. Int J Pharm X. 2024. PMID: 39309631 Free PMC article. Review.
-
Editorial for the Special Issue on the Insights and Advancements in Microfluidics.Micromachines (Basel). 2017 Aug 17;8(8):254. doi: 10.3390/mi8080254. Micromachines (Basel). 2017. PMID: 30400442 Free PMC article.
-
Simple Design for Membrane-Free Microphysiological Systems to Model the Blood-Tissue Barriers.bioRxiv [Preprint]. 2023 Oct 23:2023.10.20.563328. doi: 10.1101/2023.10.20.563328. bioRxiv. 2023. Update in: Organs Chip. 2023 Dec;5:100032. doi: 10.1016/j.ooc.2023.100032. PMID: 37961220 Free PMC article. Updated. Preprint.
-
Toward the Effective Bioengineering of a Pathological Tissue for Cardiovascular Disease Modeling: Old Strategies and New Frontiers for Prevention, Diagnosis, and Therapy.Front Cardiovasc Med. 2021 Mar 4;7:591583. doi: 10.3389/fcvm.2020.591583. eCollection 2020. Front Cardiovasc Med. 2021. PMID: 33748193 Free PMC article. Review.
-
Microfluidic investigation for shear-stress-mediated repair of dysglycemia-induced endothelial cell damage.Mechanobiol Med. 2024 Apr 29;2(3):100069. doi: 10.1016/j.mbm.2024.100069. eCollection 2024 Sep. Mechanobiol Med. 2024. PMID: 40395495 Free PMC article. Review.
References
-
- Dull R.O., Davies P.F. Flow modulation of agonist (ATP)-response (Ca2+) coupling in vascular endothelial cells. Am. J. Physiol. 1991;261:149–154. - PubMed
-
- Mo M., Eskin S.G., Schilling W.P. Flow-induced changes in Ca2+ signaling of vascular endothelial cells: Effect of shear stress and ATP. Am. J. Physiol. 1991;260:1698–1707. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

