Quantitative proteomic analysis of aqueous humor from patients with drusen and reticular pseudodrusen in age-related macular degeneration
- PMID: 30404605
- PMCID: PMC6222993
- DOI: 10.1186/s12886-018-0941-9
Quantitative proteomic analysis of aqueous humor from patients with drusen and reticular pseudodrusen in age-related macular degeneration
Abstract
Background: To identify novel biomarkers related to the pathogenesis of dry age-related macular degeneration (AMD), we adopted a human retinal pigment epithelial (RPE) cell culture model that mimics some features of dry AMD including the accumulation of intra- and sub-RPE deposits. Then, we investigated the aqueous humor (AH) proteome using a data-independent acquisition method (sequential window acquisition of all theoretical fragment ion mass spectrometry) for dry AMD patients and controls.
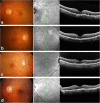
Methods: After uniformly pigmented polarized monolayers of human fetal primary RPE (hfRPE) cells were established, the cells were exposed to 4-hydroxy-2-nonenal (4-HNE), followed by Western blotting, immunofluorescence analysis and ELISA of cells or conditioned media for several proteins of interest. Data-dependent acquisition for identification of the AH proteome and SWATH-based mass spectrometry were performed for 11 dry AMD patients according to their phenotypes (including soft drusen and reticular pseudodrusen [RPD]) and 2 controls (3 groups).
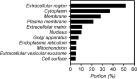
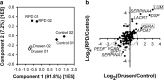
Results: Increased intra- and sub-RPE deposits were observed in 4-HNE-treated hfRPE cells compared with control cultures based on APOA1, cathepsin D, and clusterin immunoreactivity. Additionally, the differential abundance of proteins in apical and basal chambers with or without 4-HNE treatment confirmed the polarized secretion of proteins from hfRPE cells. A total of 119 proteins were quantified in dry AMD patients and controls by SWATH-MS. Sixty-five proteins exhibited significantly altered abundance among the three groups. A two-dimensional principal component analysis plot was generated to identify typical proteins related to the pathogenesis of dry AMD. Among the identified proteins, eight proteins, including APOA1, CFHR2, and CLUS, were previously considered major components or regulators of drusen. Three proteins (SERPINA4, LUM, and KERA proteins) have not been previously described as components of drusen or as being related to dry AMD. Interestingly, the LUM and KERA proteins, which are related to extracellular matrix organization, were upregulated in both RPD and soft drusen.
Conclusions: Differential protein expression in the AH between patients with drusen and RPD was quantified using SWATH-MS in the present study. Detailed proteomic analyses of dry AMD patients might provide insights into the in vivo biology of drusen and RPD.
Keywords: Age-related macular degeneration; Complement; Drusen; Reticular pseudodrusen; SWATH-MS.
Conflict of interest statement
Ethics approval and consent to participate
The study followed the tenets of the Declaration of Helsinki and was approved by the Ethics Committee of Konkuk University Medical Center (KUH1100057). Written informed consent was obtained from the patients.
Consent for publication
Not applicable.
Competing interests
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



References
-
- Rofagha S, Bhisitkul RB, Boyer DS, Sadda SR, Zhang K. Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: a multicenter cohort study (SEVEN-UP). Ophthalmology. 2013. - PubMed
-
- Bloch SB, Larsen M, Munch IC. Incidence of legal blindness from age-related macular degeneration in Denmark: year 2000 to 2010. Am J Ophthalmol. 2012. - PubMed
-
- Alten F, Eter N. Current knowledge on reticular pseudodrusen in age-related macular degeneration. Br J Ophthalmol. 2015. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

