The initial deficiency of protein processing and flavonoids biosynthesis were the main mechanisms for the male sterility induced by SX-1 in Brassica napus
- PMID: 30404610
- PMCID: PMC6223035
- DOI: 10.1186/s12864-018-5203-y
The initial deficiency of protein processing and flavonoids biosynthesis were the main mechanisms for the male sterility induced by SX-1 in Brassica napus
Abstract
Background: Rapeseed (Brassica napus) is an important oil seed crop in the Brassicaceae family. Chemical induced male sterility (CIMS) is one of the widely used method to produce the hybrids in B. napus. Identification of the key genes and pathways that involved in CIMS were important to understand the underlying molecular mechanism. In the present report, a multi-omics integrative analysis, including of the proteomic, transcriptomic and miRNAs, combined with morphological and physiological analysis were conducted.
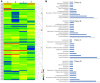
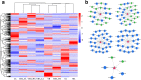
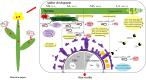
Results: Earlier degeneration of the tapetosomes and elaioplasts, aberrantly stacking in tapetal cells and incompletely deposition in tryphine of pollen wall were observed in chemical hybridization agent (CHA) of SX-1 treated B. napus through SEM and TEM analysis. It was revealed that the deficiencies in protein processing in endoplasmic reticulum (ER) and flavonoids biosynthesis were occurred at early stage in the SX-1 treated materials. Subsequently, plant hormone signal transduction, biosynthesis of amino acids, fatty acids and steroid in anther at later stages were identified down-regulated after SX-1 treatment. 144 transcript factors (TFs) were also indentified to down-regulated at early stage, which suggested the early regulation in anther and pollen wall development were disordered in CHA treated B. napus. In addition, 7 important miRNAs were identified and 2 of the predicted target genes of miRNAs were Rf-like genes.
Conclusions: Taken together, an interaction network of candidate genes and the putative metabolism pathways were constructed based on the multi-omics integrative analysis, it provided a new insight into the male sterility induced by CHA of SX-1 in B. napus.
Keywords: Brassica napus; Chemical hybridization agent; Flavonoids biosynthesis; Male sterility; Protein processing; Proteome; Transcript factor; Transcriptome; Tryphine; miRNA.
Conflict of interest statement
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures





References
-
- Saxena KB, Sultana R, Mallikarjuna N, Saxena RK, Kumar RV, Sawargaonkar SL, Varshney RK. Male-sterility systems in pigeonpea and their role in enhancing yield. Plant Breed. 2010;129:125–134. doi: 10.1111/j.1439-0523.2009.01752.x. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

