Infection and Functional Modulation of Human Monocytes and Macrophages by Varicella-Zoster Virus
- PMID: 30404793
- PMCID: PMC6340020
- DOI: 10.1128/JVI.01887-18
Infection and Functional Modulation of Human Monocytes and Macrophages by Varicella-Zoster Virus
Abstract
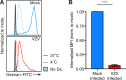
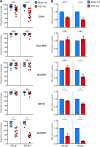
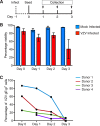
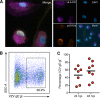
Varicella-zoster virus (VZV) is associated with viremia during primary infection that is presumed to stem from infection of circulating immune cells. While VZV has been shown to be capable of infecting a number of different subsets of circulating immune cells, such as T cells, dendritic cells, and NK cells, less is known about the interaction between VZV and monocytes. Here, we demonstrate that blood-derived human monocytes are permissive to VZV replication in vitro VZV-infected monocytes exhibited each temporal class of VZV gene expression, as evidenced by immunofluorescent staining. VZV virions were observed on the cell surface and viral nucleocapsids were observed in the nucleus of VZV-infected monocytes by scanning electron microscopy. In addition, VZV-infected monocytes were able to transfer infectious virus to human fibroblasts. Infected monocytes displayed impaired dextran-mediated endocytosis, and cell surface immunophenotyping revealed the downregulation of CD14, HLA-DR, CD11b, and the macrophage colony-stimulating factor (M-CSF) receptor. Analysis of the impact of VZV infection on M-CSF-stimulated monocyte-to-macrophage differentiation demonstrated the loss of cell viability, indicating that VZV-infected monocytes were unable to differentiate into viable macrophages. In contrast, macrophages differentiated from monocytes prior to exposure to VZV were highly permissive to infection. This study defines the permissiveness of these myeloid cell types to productive VZV infection and identifies the functional impairment of VZV-infected monocytes.IMPORTANCE Primary VZV infection results in the widespread dissemination of the virus throughout the host. Viral transportation is known to be directly influenced by susceptible immune cells in the circulation. Moreover, infection of immune cells by VZV results in attenuation of the antiviral mechanisms used to control infection and limit spread. Here, we provide evidence that human monocytes, which are highly abundant in the circulation, are permissive to productive VZV infection. Furthermore, monocyte-derived macrophages were also highly permissive to VZV infection, although VZV-infected monocytes were unable to differentiate into macrophages. Exploring the relationships between VZV and permissive immune cells, such as human monocytes and macrophages, elucidates novel immune evasion strategies and provides further insight into the control that VZV has over the immune system.
Keywords: varicella-zoster virus.
Copyright © 2019 American Society for Microbiology.
Figures
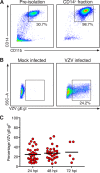

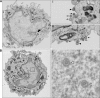
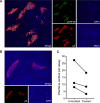
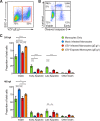
References
-
- Arvin A. 2001. Varicella-zoster virus In Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE (ed), Fields virology, 4th ed Lippincott Williams & Wilkins, Philadelphia, PA.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

