Cell-to-Cell Transmission Is the Main Mechanism Supporting Bovine Viral Diarrhea Virus Spread in Cell Culture
- PMID: 30404802
- PMCID: PMC6340029
- DOI: 10.1128/JVI.01776-18
Cell-to-Cell Transmission Is the Main Mechanism Supporting Bovine Viral Diarrhea Virus Spread in Cell Culture
Abstract
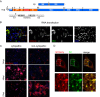
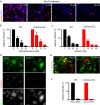
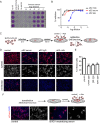
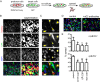
After initiation of an infective cycle, spread of virus infection can occur in two fundamentally different ways: (i) viral particles can be released into the external environment and diffuse through the extracellular space until they interact with a new host cell, and (ii) virions can remain associated with infected cells, promoting the direct passage between infected and uninfected cells that is referred to as direct cell-to-cell transmission. Although evidence of cell-associated transmission has accumulated for many different viruses, the ability of members of the genus Pestivirus to use this mode of transmission has not been reported. In the present study, we used a novel recombinant virus expressing the envelope glycoprotein E2 fused to mCherry fluorescent protein to monitor the spreading of bovine viral diarrhea virus (BVDV) (the type member of the pestiviruses) infection. To demonstrate direct cell-to-cell transmission of BVDV, we developed a cell coculture system that allowed us to prove direct transmission from infected to uninfected cells in the presence of neutralizing antibodies. This mode of transmission requires cell-cell contacts and clathrin-mediated receptor-dependent endocytosis. Notably, it overcomes antibody blocking of the BVDV receptor CD46, indicating that cell-to-cell transmission of the virus involves the engagement of coreceptors on the target cell.IMPORTANCE BVDV causes one of the most economically important viral infections for the cattle industry. The virus is able to cross the placenta and infect the fetus, leading to the birth of persistently infected animals, which are reservoirs for the spread of BVDV. The occurrence of persistent infection has hampered the efficacy of vaccination because it requires eliciting levels of protection close to sterilizing immunity to prevent fetal infections. While vaccination prevents disease, BVDV can be detected if animals with neutralizing antibodies are challenged with the virus. Virus cell-to-cell transmission allows the virus to overcome barriers to free virus dissemination, such as antibodies or epithelial barriers. Here we show that BVDV exploits cell-cell contacts to propagate infection in a process that is resistant to antibody neutralization. Our results provide new insights into the mechanisms underlying the pathogenesis of BVDV infection and can aid in the design of effective control strategies.
Keywords: CD46; E2; bovine viral diarrhea virus; cell-to-cell transmission; endocytosis; pestiviruses; reporter genes; surface receptor; virus entry.
Copyright © 2019 American Society for Microbiology.
Figures


Similar articles
-
A CRISPR/Cas9 Generated Bovine CD46-knockout Cell Line-A Tool to Elucidate the Adaptability of Bovine Viral Diarrhea Viruses (BVDV).Viruses. 2020 Aug 6;12(8):859. doi: 10.3390/v12080859. Viruses. 2020. PMID: 32781607 Free PMC article.
-
HoBi-Like Virus RNA Detected in Foetuses Following Challenge of Pregnant Cows that had Previously Given Birth to Calves Persistently Infected with Bovine Viral Diarrhoea Virus.Transbound Emerg Dis. 2017 Oct;64(5):1624-1632. doi: 10.1111/tbed.12556. Epub 2016 Sep 11. Transbound Emerg Dis. 2017. PMID: 27615437
-
Expression of Bovine Viral Diarrhea Virus Envelope Glycoprotein E2 in Yeast Pichia pastoris and its Application to an ELISA for Detection of BVDV Neutralizing Antibodies in Cattle.J Immunoassay Immunochem. 2015;36(6):639-54. doi: 10.1080/15321819.2015.1032305. J Immunoassay Immunochem. 2015. PMID: 25837831
-
[Bovine diarrhea virus: an update].Rev Argent Microbiol. 1997 Jan-Mar;29(1):47-61. Rev Argent Microbiol. 1997. PMID: 9229725 Review. Spanish.
-
Impact of species and subgenotypes of bovine viral diarrhea virus on control by vaccination.Anim Health Res Rev. 2015 Jun;16(1):40-54. doi: 10.1017/S1466252315000079. Anim Health Res Rev. 2015. PMID: 26050571 Review.
Cited by
-
Comprehensive analysis of lncRNA expression profiles in cytopathic biotype BVDV-infected MDBK cells provides an insight into biological contexts of host-BVDV interactions.Virulence. 2021 Dec;12(1):20-34. doi: 10.1080/21505594.2020.1857572. Virulence. 2021. PMID: 33258421 Free PMC article.
-
Real Time Analysis of Bovine Viral Diarrhea Virus (BVDV) Infection and Its Dependence on Bovine CD46.Viruses. 2020 Jan 17;12(1):116. doi: 10.3390/v12010116. Viruses. 2020. PMID: 31963539 Free PMC article.
-
Porcine Complement Regulatory Protein CD46 Is a Major Receptor for Atypical Porcine Pestivirus but Not for Classical Swine Fever Virus.J Virol. 2021 Apr 12;95(9):e02186-20. doi: 10.1128/JVI.02186-20. Print 2021 Apr 12. J Virol. 2021. PMID: 33568504 Free PMC article.
-
Viral Traits and Cellular Knock-Out Genotype Affect Dependence of BVDV on Bovine CD46.Pathogens. 2021 Dec 14;10(12):1620. doi: 10.3390/pathogens10121620. Pathogens. 2021. PMID: 34959575 Free PMC article.
-
Porcine deltacoronavirus resists antibody neutralization through cell-to-cell transmission.Emerg Microbes Infect. 2023 Dec;12(1):2207688. doi: 10.1080/22221751.2023.2207688. Emerg Microbes Infect. 2023. PMID: 37125733 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

