Murine Cytomegalovirus Glycoprotein O Promotes Epithelial Cell Infection In Vivo
- PMID: 30404805
- PMCID: PMC6340042
- DOI: 10.1128/JVI.01378-18
Murine Cytomegalovirus Glycoprotein O Promotes Epithelial Cell Infection In Vivo
Abstract
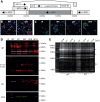
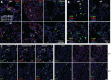
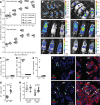
Cytomegaloviruses (CMVs) establish systemic infections across diverse cell types. Glycoproteins that alter tropism can potentially guide their spread. Glycoprotein O (gO) is a nonessential fusion complex component of both human CMV (HCMV) and murine CMV (MCMV). We tested its contribution to MCMV spread from the respiratory tract. In vitro, MCMV lacking gO poorly infected fibroblasts and epithelial cells. Cell binding was intact, but penetration was delayed. In contrast, myeloid infection was preserved, and in the lungs, where myeloid and type 2 alveolar epithelial cells are the main viral targets, MCMV lacking gO showed a marked preference for myeloid infection. Its poor epithelial cell infection was associated with poor primary virus production and reduced virulence. Systemic spread, which proceeds via infected CD11c+ myeloid cells, was initially intact but then diminished, because less epithelial infection led ultimately to less myeloid infection. Thus, the tight linkage between peripheral and systemic MCMV infections gave gO-dependent infection a central role in host colonization.IMPORTANCE Human cytomegalovirus is a leading cause of congenital disease. This reflects its capacity for systemic spread. A vaccine is needed, but the best viral targets are unclear. Attention has focused on the virion membrane fusion complex. It has 2 forms, so we need to know what each contributes to host colonization. One includes the virion glycoprotein O. We used murine cytomegalovirus, which has equivalent fusion complexes, to determine the importance of glycoprotein O after mucosal infection. We show that it drives local virus replication in epithelial cells. It was not required to infect myeloid cells, which establish systemic infection, but poor local replication reduced systemic spread as a secondary effect. Therefore, targeting glycoprotein O of human cytomegalovirus has the potential to reduce both local and systemic infections.
Keywords: cytomegalovirus; dissemination; glycoproteins; host colonization; pathogenesis; tropism.
Copyright © 2019 American Society for Microbiology.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

