Induction and Suppression of NF-κB Signalling by a DNA Virus of Drosophila
- PMID: 30404807
- PMCID: PMC6340046
- DOI: 10.1128/JVI.01443-18
Induction and Suppression of NF-κB Signalling by a DNA Virus of Drosophila
Abstract
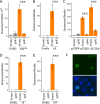
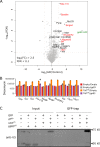
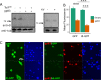
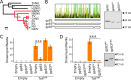
Interactions between the insect immune system and RNA viruses have been extensively studied in Drosophila, in which RNA interference, NF-κB, and JAK-STAT pathways underlie antiviral immunity. In response to RNA interference, insect viruses have convergently evolved suppressors of this pathway that act by diverse mechanisms to permit viral replication. However, interactions between the insect immune system and DNA viruses have received less attention, primarily because few Drosophila-infecting DNA virus isolates are available. In this study, we used a recently isolated DNA virus of Drosophila melanogaster, Kallithea virus (KV; family Nudiviridae), to probe known antiviral immune responses and virus evasion tactics in the context of DNA virus infection. We found that fly mutants for RNA interference and immune deficiency (Imd), but not Toll, pathways are more susceptible to Kallithea virus infection. We identified the Kallithea virus-encoded protein gp83 as a potent inhibitor of Toll signalling, suggesting that Toll mediates antiviral defense against Kallithea virus infection but that it is suppressed by the virus. We found that Kallithea virus gp83 inhibits Toll signalling through the regulation of NF-κB transcription factors. Furthermore, we found that gp83 of the closely related Drosophila innubila nudivirus (DiNV) suppresses D. melanogaster Toll signalling, suggesting an evolutionarily conserved function of Toll in defense against DNA viruses. Together, these results provide a broad description of known antiviral pathways in the context of DNA virus infection and identify the first Toll pathway inhibitor in a Drosophila virus, extending the known diversity of insect virus-encoded immune inhibitors.IMPORTANCE Coevolution of multicellular organisms and their natural viruses may lead to an intricate relationship in which host survival requires effective immunity and virus survival depends on evasion of such responses. Insect antiviral immunity and reciprocal virus immunosuppression tactics have been well studied in Drosophila melanogaster, primarily during RNA, but not DNA, virus infection. Therefore, we describe interactions between a recently isolated Drosophila DNA virus (Kallithea virus [KV]) and immune processes known to control RNA viruses, such as RNA interference (RNAi) and Imd pathways. We found that KV suppresses the Toll pathway and identified gp83 as a KV-encoded protein that underlies this suppression. This immunosuppressive ability is conserved in another nudivirus, suggesting that the Toll pathway has conserved antiviral activity against DNA nudiviruses, which have evolved suppressors in response. Together, these results indicate that DNA viruses induce and suppress NF-κB responses, and they advance the application of KV as a model to study insect immunity.
Keywords: Drosophila melanogaster; NF-κB; RNA interference; immune suppression; innate immunity.
Copyright © 2019 American Society for Microbiology.
Figures
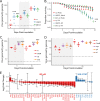
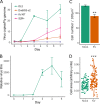
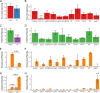
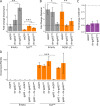
Similar articles
-
Protein-coding circular RNA enhances antiviral immunity via JAK/STAT pathway in Drosophila.mBio. 2024 Sep 11;15(9):e0146924. doi: 10.1128/mbio.01469-24. Epub 2024 Aug 19. mBio. 2024. PMID: 39158293 Free PMC article.
-
Drosophila melanogaster Toll-9 elicits antiviral immunity against Drosophila C virus.J Virol. 2025 Jun 17;99(6):e0221424. doi: 10.1128/jvi.02214-24. Epub 2025 May 14. J Virol. 2025. PMID: 40366172 Free PMC article.
-
The antiviral role of NF-κB-mediated immune responses and their antagonism by viruses in insects.J Gen Virol. 2022 May;103(5). doi: 10.1099/jgv.0.001741. J Gen Virol. 2022. PMID: 35510990
-
Insect antiviral innate immunity: pathways, effectors, and connections.J Mol Biol. 2013 Dec 13;425(24):4921-36. doi: 10.1016/j.jmb.2013.10.006. Epub 2013 Oct 9. J Mol Biol. 2013. PMID: 24120681 Free PMC article. Review.
-
Sensing and signalling viral infection in drosophila.Dev Comp Immunol. 2021 Apr;117:103985. doi: 10.1016/j.dci.2020.103985. Epub 2020 Dec 23. Dev Comp Immunol. 2021. PMID: 33358662 Review.
Cited by
-
IIV-6 Inhibits NF-κB Responses in Drosophila.Viruses. 2019 May 1;11(5):409. doi: 10.3390/v11050409. Viruses. 2019. PMID: 31052481 Free PMC article.
-
The discovery, distribution, and diversity of DNA viruses associated with Drosophila melanogaster in Europe.Virus Evol. 2021 Apr 1;7(1):veab031. doi: 10.1093/ve/veab031. eCollection 2021 Jan. Virus Evol. 2021. PMID: 34408913 Free PMC article.
-
Drosophila caspases as guardians of host-microbe interactions.Cell Death Differ. 2023 Feb;30(2):227-236. doi: 10.1038/s41418-022-01038-4. Epub 2022 Jul 9. Cell Death Differ. 2023. PMID: 35810247 Free PMC article. Review.
-
A DNA virus-encoded immune antagonist fully masks the potent antiviral activity of RNAi in Drosophila.Proc Natl Acad Sci U S A. 2019 Nov 26;116(48):24296-24302. doi: 10.1073/pnas.1909183116. Epub 2019 Nov 11. Proc Natl Acad Sci U S A. 2019. PMID: 31712431 Free PMC article.
-
Pseudorabies Virus ICP0 Abolishes Tumor Necrosis Factor Alpha-Induced NF-κB Activation by Degrading P65.Viruses. 2022 May 2;14(5):954. doi: 10.3390/v14050954. Viruses. 2022. PMID: 35632696 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

