Resolution of sickle cell disease-associated inflammation and tissue damage with 17 R-resolvin D1
- PMID: 30404812
- PMCID: PMC6337877
- DOI: 10.1182/blood-2018-07-865378
Resolution of sickle cell disease-associated inflammation and tissue damage with 17 R-resolvin D1
Abstract
Resolvins (Rvs), endogenous lipid mediators, play a key role in the resolution of inflammation. Sickle cell disease (SCD), a genetic disorder of hemoglobin, is characterized by inflammatory and vaso-occlusive pathologies. We document altered proresolving events following hypoxia/reperfusion in humanized SCD mice. We demonstrate novel protective actions of 17R-resolvin D1 (17R-RvD1; 7S, 8R, 17R-trihydroxy-4Z, 9E, 11E, 13Z, 15E, 19Z-docosahexaenoic acid) in reducing ex vivo human SCD blood leukocyte recruitment by microvascular endothelial cells and in vivo neutrophil adhesion and transmigration. In SCD mice exposed to hypoxia/reoxygenation, oral administration of 17R -RvD1 reduces systemic/local inflammation and vascular dysfunction in lung and kidney. The mechanism of action of 17R-RvD1 involves (1) enhancement of SCD erythrocytes and polymorphonuclear leukocyte efferocytosis, (2) blunting of NF-κB activation, and (3) a reduction in inflammatory cytokines, vascular activation markers, and E-selectin expression. Thus, 17R-RvD1 might represent a new therapeutic strategy for the inflammatory vasculopathy of SCD.
© 2019 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures

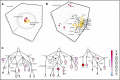
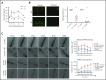
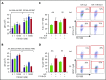
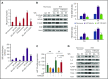
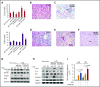
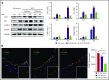
Comment in
-
Resolving inflammation and pain of sickle cell.Blood. 2019 Jan 17;133(3):190-191. doi: 10.1182/blood-2018-11-886259. Blood. 2019. PMID: 30655305 Free PMC article.
References
-
- De Franceschi L, Cappellini MD, Olivieri O. Thrombosis and sickle cell disease. Semin Thromb Hemost. 2011;37(3):226-236. - PubMed
-
- Vinchi F, De Franceschi L, Ghigo A, et al. Hemopexin therapy improves cardiovascular function by preventing heme-induced endothelial toxicity in mouse models of hemolytic diseases. Circulation. 2013;127(12):1317-1329. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

