Cytokine exocytosis and JAK/STAT activation in the Drosophila ovary requires the vesicle trafficking regulator α-Snap
- PMID: 30404830
- PMCID: PMC6288073
- DOI: 10.1242/jcs.217638
Cytokine exocytosis and JAK/STAT activation in the Drosophila ovary requires the vesicle trafficking regulator α-Snap
Abstract
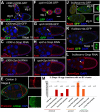
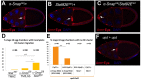
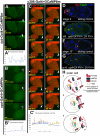
How vesicle trafficking components actively contribute to regulation of paracrine signaling is unclear. We genetically uncovered a requirement for α-soluble NSF attachment protein (α-Snap) in the activation of the Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway during Drosophila egg development. α-Snap, a well-conserved vesicle trafficking regulator, mediates association of N-ethylmaleimide-sensitive factor (NSF) and SNAREs to promote vesicle fusion. Depletion of α-Snap or the SNARE family member Syntaxin1A in epithelia blocks polar cells maintenance and prevents specification of motile border cells. Blocking apoptosis rescues polar cell maintenance in α-Snap-depleted egg chambers, indicating that the lack of border cells in mutants is due to impaired signaling. Genetic experiments implicate α-Snap and NSF in secretion of a STAT-activating cytokine. Live imaging suggests that changes in intracellular Ca2+ are linked to this event. Our data suggest a cell-type specific requirement for particular vesicle trafficking components in regulated exocytosis during development. Given the central role for STAT signaling in immunity, this work may shed light on regulation of cytokine release in humans.
Keywords: Cell fate specification; Drosophila oogenesis; Exocytosis; JAK/STAT signaling.
© 2018. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures

Similar articles
-
Drosophila Jak/STAT Signaling: Regulation and Relevance in Human Cancer and Metastasis.Int J Mol Sci. 2018 Dec 14;19(12):4056. doi: 10.3390/ijms19124056. Int J Mol Sci. 2018. PMID: 30558204 Free PMC article. Review.
-
Socs36E limits STAT signaling via Cullin2 and a SOCS-box independent mechanism in the Drosophila egg chamber.Mech Dev. 2015 Nov;138 Pt 3:313-27. doi: 10.1016/j.mod.2015.08.003. Epub 2015 Aug 13. Mech Dev. 2015. PMID: 26277564
-
α-SNAP prevents docking of the acrosome during sperm exocytosis because it sequesters monomeric syntaxin.PLoS One. 2011;6(7):e21925. doi: 10.1371/journal.pone.0021925. Epub 2011 Jul 18. PLoS One. 2011. PMID: 21789195 Free PMC article.
-
A novel site of action for alpha-SNAP in the SNARE conformational cycle controlling membrane fusion.Mol Biol Cell. 2008 Mar;19(3):776-84. doi: 10.1091/mbc.e07-05-0498. Epub 2007 Dec 19. Mol Biol Cell. 2008. PMID: 18094056 Free PMC article.
-
Neurotransmitter release - four years of SNARE complexes.Curr Opin Neurobiol. 1997 Jun;7(3):310-5. doi: 10.1016/s0959-4388(97)80057-8. Curr Opin Neurobiol. 1997. PMID: 9232812 Review.
Cited by
-
Corazonin Neurons Contribute to Dimorphic Ethanol Sedation Sensitivity in Drosophila melanogaster.Front Neural Circuits. 2022 Jun 22;16:702901. doi: 10.3389/fncir.2022.702901. eCollection 2022. Front Neural Circuits. 2022. PMID: 35814486 Free PMC article.
-
Platelet-released extracellular vesicles: the effects of thrombin activation.Cell Mol Life Sci. 2022 Mar 14;79(3):190. doi: 10.1007/s00018-022-04222-4. Cell Mol Life Sci. 2022. PMID: 35288766 Free PMC article.
-
Drosophila Jak/STAT Signaling: Regulation and Relevance in Human Cancer and Metastasis.Int J Mol Sci. 2018 Dec 14;19(12):4056. doi: 10.3390/ijms19124056. Int J Mol Sci. 2018. PMID: 30558204 Free PMC article. Review.
References
-
- Andreeva A. V., Kutuzov M. A., Vaiskunaite R., Profirovic J., Meigs T. E., Predescu S., Malik A. B. and Voyno-Yasenetskaya T. (2005). G alpha12 interaction with alphaSNAP induces VE-cadherin localization at endothelial junctions and regulates barrier function. J. Biol. Chem. 280, 30376-30383. 10.1074/jbc.M502844200 - DOI - PubMed
-
- Arcos A., Paola M., Gianetti D., Acuña D., Velásquez Z. D., Miró M. P., Toro G., Hinrichsen B., Munoz R. I., Lin Y. et al. (2017). alpha-SNAP is expressed in mouse ovarian granulosa cells and plays a key role in folliculogenesis and female fertility. Sci. Rep. 7, 11765 10.1038/s41598-017-12292-9 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

