Alpha-synuclein fibrils recruit TBK1 and OPTN to lysosomal damage sites and induce autophagy in microglial cells
- PMID: 30404831
- PMCID: PMC6518333
- DOI: 10.1242/jcs.226241
Alpha-synuclein fibrils recruit TBK1 and OPTN to lysosomal damage sites and induce autophagy in microglial cells
Abstract
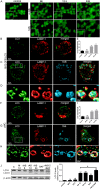
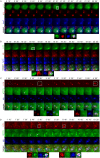
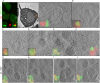
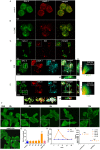
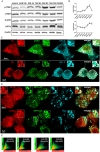
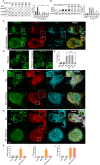
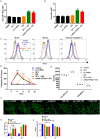
Autophagic dysfunction and protein aggregation have been linked to several neurodegenerative disorders, but the exact mechanisms and causal connections are not clear and most previous work was done in neurons and not in microglial cells. Here, we report that exogenous fibrillary, but not monomeric, alpha-synuclein (AS, also known as SNCA) induces autophagy in microglial cells. We extensively studied the dynamics of this response using both live-cell imaging and correlative light-electron microscopy (CLEM), and found that it correlates with lysosomal damage and is characterised by the recruitment of the selective autophagy-associated proteins TANK-binding kinase 1 (TBK1) and optineurin (OPTN) to ubiquitylated lysosomes. In addition, we observed that LC3 (MAP1LC3B) recruitment to damaged lysosomes was dependent on TBK1 activity. In these fibrillar AS-treated cells, autophagy inhibition impairs mitochondrial function and leads to microglial cell death. Our results suggest that microglial autophagy is induced in response to lysosomal damage caused by persistent accumulation of AS fibrils. Importantly, triggering of the autophagic response appears to be an attempt at lysosomal quality control and not for engulfment of fibrillar AS.This article has an associated First Person interview with the first author of the paper.
Keywords: Alpha-synuclein; Autophagy; Cell death; Lysosomes; Microglia.
© 2018. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures

References
-
- Aits S., Kricker J., Liu B., Ellegaard A.-M., Hämälistö S., Tvingsholm S., Corcelle-Termeau E., Høgh S., Farkas T., Holm Jonassen A. et al. (2015). Sensitive detection of lysosomal membrane permeabilization by lysosomal galectin puncta assay. Autophagy 11, 1408-1424. 10.1080/15548627.2015.1063871 - DOI - PMC - PubMed
-
- Axe E. L., Walker S. A., Manifava M., Chandra P., Roderick H. L., Habermann A., Griffiths G. and Ktistakis N. T. (2008). Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3-phosphate and dynamically connected to the endoplasmic reticulum. J. Cell Biol. 182, 685-701. 10.1083/jcb.200803137 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

