Intraspecific brain size variation between coexisting sunfish ecotypes
- PMID: 30404883
- PMCID: PMC6235049
- DOI: 10.1098/rspb.2018.1971
Intraspecific brain size variation between coexisting sunfish ecotypes
Abstract
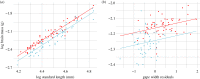
Variation in spatial complexity and foraging requirements between habitats can impose different cognitive demands on animals that may influence brain size. However, the relationship between ecologically related cognitive performance and brain size is not well established. We test whether variation in relative brain size and brain region size is associated with habitat use within a population of pumpkinseed sunfish composed of different ecotypes that inhabit either the structurally complex shoreline littoral habitat or simpler open-water pelagic habitat. Sunfish using the littoral habitat have on average 8.3% larger brains than those using the pelagic habitat. We found little difference in the proportional sizes of five brain regions between ecotypes. The results suggest that cognitive demands on sunfish may be reduced in the pelagic habitat given no habitat-specific differences in body condition. They also suggest that either a short divergence time or physiological processes may constrain changes to concerted, global modifications of brain size between sunfish ecotypes.
Keywords: adaptive divergence; brain size; cognitive ecology; habitat; plasticity; sunfish.
© 2018 The Author(s).
Conflict of interest statement
We declare no competing interests.
Figures
References
-
- Striedter GF. 2005. Principles of brain evolution. Sunderland, MA: Sinauer Associates.
-
- Mayr E. 1982. The growth of biological thought: diversity, evolution, and inheritance. Cambridge, MA: Harvard University Press.
-
- Kondoh M. 2010. Linking learning adaptation to trophic interactions: a brain size-based approach. Funct. Ecol. 24, 35–43. ( 10.1111/j.1365-2435.2009.01631.x) - DOI
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
