A flexible organic reflectance oximeter array
- PMID: 30404911
- PMCID: PMC6255203
- DOI: 10.1073/pnas.1813053115
A flexible organic reflectance oximeter array
Abstract
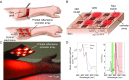
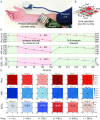
Transmission-mode pulse oximetry, the optical method for determining oxygen saturation in blood, is limited to only tissues that can be transilluminated, such as the earlobes and the fingers. The existing sensor configuration provides only single-point measurements, lacking 2D oxygenation mapping capability. Here, we demonstrate a flexible and printed sensor array composed of organic light-emitting diodes and organic photodiodes, which senses reflected light from tissue to determine the oxygen saturation. We use the reflectance oximeter array beyond the conventional sensing locations. The sensor is implemented to measure oxygen saturation on the forehead with 1.1% mean error and to create 2D oxygenation maps of adult forearms under pressure-cuff-induced ischemia. In addition, we present mathematical models to determine oxygenation in the presence and absence of a pulsatile arterial blood signal. The mechanical flexibility, 2D oxygenation mapping capability, and the ability to place the sensor in various locations make the reflectance oximeter array promising for medical sensing applications such as monitoring of real-time chronic medical conditions as well as postsurgery recovery management of tissues, organs, and wounds.
Keywords: bioelectronics; flexible electronics; organic electronics; oximetry; wearable sensors.
Copyright © 2018 the Author(s). Published by PNAS.
Conflict of interest statement
Conflict of interest statement: A provisional patent application has been filed based on the technology described in this work.
Figures



References
-
- Webster JG. Design of Pulse Oximeters. Taylor & Francis; New York: 1997.
-
- Nakajimi S, Hirai H, Takase H, Kuze A, Aoyagi S. New pulsed-type earpiece oximeter. Respiration Circ. 1975;23:709–713. - PubMed
-
- Someya T, Bao Z, Malliaras GG. The rise of plastic bioelectronics. Nature. 2016;540:379–385. - PubMed
-
- Choi S, Lee H, Ghaffari R, Hyeon T, Kim DH. Recent advances in flexible and stretchable bio-electronic devices integrated with nanomaterials. Adv Mater. 2016;28:4203–4218. - PubMed
-
- Trung TQ, Lee NE. Flexible and stretchable physical sensor integrated platforms for wearable human-activity monitoring and personal healthcare. Adv Mater. 2016;28:4338–4372. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

