The Buoyancy of Cryptococcus neoformans Is Affected by Capsule Size
- PMID: 30404928
- PMCID: PMC6222054
- DOI: 10.1128/mSphere.00534-18
The Buoyancy of Cryptococcus neoformans Is Affected by Capsule Size
Abstract
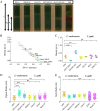
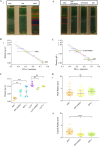
Cryptococcus neoformans is an environmental pathogenic fungus with a worldwide geographical distribution that is responsible for hundreds of thousands of human cryptococcosis cases each year. During infection, the yeast undergoes a morphological transformation involving capsular enlargement that increases microbial volume. To understand the factors that play a role in environmental dispersal of C. neoformans and C. gattii, we evaluated the cell density of Cryptococcus using Percoll isopycnic gradients. We found differences in the cell densities of strains belonging to C. neoformans and C. gattii species complexes. The buoyancy of C. neoformans strains varied depending on growth medium. In minimal medium, the cryptococcal capsule made a major contribution to the cell density such that cells with larger capsules had lower density than those with smaller capsules. Removing the capsule, by chemical or mechanical methods, increased the C. neoformans cell density and reduced buoyancy. Melanization of the C. neoformans cell wall, which also contributes to virulence, produced a small but consistent increase in cell density. Encapsulated C. neoformans sedimented much more slowly in seawater as its density approached the density of water. Our results suggest a new function for the capsule whereby it can function as a flotation device to facilitate transport and dispersion in aqueous fluids.IMPORTANCE The buoyancy of a microbial cell is an important physical characteristic that may affect its transportability in fluids and interactions with tissues during infection. The polysaccharide capsule surrounding C. neoformans is required for infection and dissemination in the host. Our results indicate that the capsule has a significant effect on reducing cryptococcal cell density, altering its sedimentation in seawater. Modulation of microbial cell density via encapsulation may facilitate dispersal for other important encapsulated pathogens.
Keywords: Cryptococcus neoformans; buoyancy; capsular polysaccharide; yeast density.
Copyright © 2018 Vij et al.
Figures




References
-
- Perfect JR. 2000. Cryptococcosis, p 79–93. In Atlas of infectious diseases. Current Medicine Group, London, United Kingdom.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
