Molecular structure of promoter-bound yeast TFIID
- PMID: 30405110
- PMCID: PMC6220335
- DOI: 10.1038/s41467-018-07096-y
Molecular structure of promoter-bound yeast TFIID
Abstract
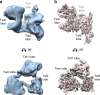
Transcription preinitiation complex assembly on the promoters of protein encoding genes is nucleated in vivo by TFIID composed of the TATA-box Binding Protein (TBP) and 13 TBP-associate factors (Tafs) providing regulatory and chromatin binding functions. Here we present the cryo-electron microscopy structure of promoter-bound yeast TFIID at a resolution better than 5 Å, except for a flexible domain. We position the crystal structures of several subunits and, in combination with cross-linking studies, describe the quaternary organization of TFIID. The compact tri lobed architecture is stabilized by a topologically closed Taf5-Taf6 tetramer. We confirm the unique subunit stoichiometry prevailing in TFIID and uncover a hexameric arrangement of Tafs containing a histone fold domain in the Twin lobe.
Conflict of interest statement
The authors declare no competing interests.
Figures


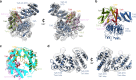

Similar articles
-
Purification of active TFIID from Saccharomyces cerevisiae. Extensive promoter contacts and co-activator function.J Biol Chem. 2004 Nov 26;279(48):49973-81. doi: 10.1074/jbc.M409849200. Epub 2004 Sep 22. J Biol Chem. 2004. PMID: 15448131
-
The architecture of human general transcription factor TFIID core complex.Nature. 2013 Jan 31;493(7434):699-702. doi: 10.1038/nature11791. Epub 2013 Jan 6. Nature. 2013. PMID: 23292512
-
Cryo-EM reveals promoter DNA binding and conformational flexibility of the general transcription factor TFIID.Structure. 2009 Nov 11;17(11):1442-52. doi: 10.1016/j.str.2009.09.007. Structure. 2009. PMID: 19913479
-
New insights into the function of transcription factor TFIID from recent structural studies.Curr Opin Genet Dev. 2011 Apr;21(2):219-24. doi: 10.1016/j.gde.2011.01.009. Epub 2011 Mar 21. Curr Opin Genet Dev. 2011. PMID: 21420851 Free PMC article. Review.
-
Promoter Recognition: Putting TFIID on the Spot.Trends Cell Biol. 2019 Sep;29(9):752-763. doi: 10.1016/j.tcb.2019.06.004. Epub 2019 Jul 9. Trends Cell Biol. 2019. PMID: 31300188 Review.
Cited by
-
Taf14 is required for the stabilization of transcription pre-initiation complex in Saccharomyces cerevisiae.Epigenetics Chromatin. 2020 May 27;13(1):24. doi: 10.1186/s13072-020-00347-7. Epigenetics Chromatin. 2020. PMID: 32460824 Free PMC article.
-
Structure and mechanism of the RNA polymerase II transcription machinery.Genes Dev. 2020 Apr 1;34(7-8):465-488. doi: 10.1101/gad.335679.119. Genes Dev. 2020. PMID: 32238450 Free PMC article. Review.
-
Differences and similarities in recognition of co-factors by Taf14.Biochim Biophys Acta Gene Regul Mech. 2023 Sep;1866(3):194961. doi: 10.1016/j.bbagrm.2023.194961. Epub 2023 Jul 22. Biochim Biophys Acta Gene Regul Mech. 2023. PMID: 37482120 Free PMC article.
-
Fine mapping of QTL conferring Cercospora leaf spot disease resistance in mungbean revealed TAF5 as candidate gene for the resistance.Theor Appl Genet. 2021 Feb;134(2):701-714. doi: 10.1007/s00122-020-03724-8. Epub 2020 Nov 13. Theor Appl Genet. 2021. PMID: 33188437
-
Transcription factor IID parks and drives preinitiation complexes at sharp or broad promoters.Trends Biochem Sci. 2023 Oct;48(10):839-848. doi: 10.1016/j.tibs.2023.07.009. Epub 2023 Aug 12. Trends Biochem Sci. 2023. PMID: 37574371 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

