Whole body PD-1 and PD-L1 positron emission tomography in patients with non-small-cell lung cancer
- PMID: 30405135
- PMCID: PMC6220188
- DOI: 10.1038/s41467-018-07131-y
Whole body PD-1 and PD-L1 positron emission tomography in patients with non-small-cell lung cancer
Abstract
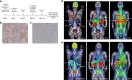
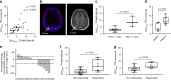
PD-L1 immunohistochemistry correlates only moderately with patient survival and response to PD-(L)1 treatment. Heterogeneity of tumor PD-L1 expression might limit the predictive value of small biopsies. Here we show that tumor PD-L1 and PD-1 expression can be quantified non-invasively using PET-CT in patients with non-small-cell lung cancer. Whole body PD-(L)1 PET-CT reveals significant tumor tracer uptake heterogeneity both between patients, as well as within patients between different tumor lesions.
Conflict of interest statement
We received a research grant for the implementation of this study from Bristol-Myers Squibb (BMS). D.Leung, R.S., S.D., W.H., P.M., D.Lipovsek, D.J.D, S.J.B., and L.M. are employees and stock holders of BMS. The remaining authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

