Loss of Sirt2 increases and prolongs a caerulein-induced pancreatitis permissive phenotype and induces spontaneous oncogenic Kras mutations in mice
- PMID: 30405152
- PMCID: PMC6220268
- DOI: 10.1038/s41598-018-34792-y
Loss of Sirt2 increases and prolongs a caerulein-induced pancreatitis permissive phenotype and induces spontaneous oncogenic Kras mutations in mice
Abstract
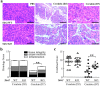
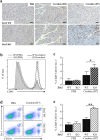
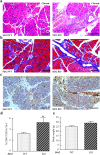
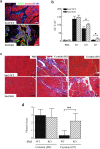
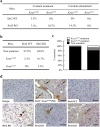
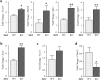
Mice lacking Sirt2 spontaneously develop tumors in multiple organs, as well as when expressed in combination with oncogenic KrasG12D, leading to pancreatic tumors. Here, we report that after caerulein-induced pancreatitis, Sirt2-deficient mice exhibited an increased inflammatory phenotype and delayed pancreatic tissue recovery. Seven days post injury, the pancreas of Sirt2-/- mice display active inflammation, whereas wild-type mice had mostly recovered. In addition, the pancreas from the Sirt2-/- mice exhibited extensive tissue fibrosis, which was still present at six weeks after exposure. The mice lacking Sirt2 also demonstrated an enhanced whole body pro-inflammatory phenotype that was most obvious with increasing age. Importantly, an accumulation of a cell population with spontaneous cancerous KrasG12D mutations was observed in the Sirt2-/- mice that is enhanced in the recovering pancreas after exposure to caerulein. Finally, transcriptome analysis of the pancreas of the Sirt2-/- mice exhibited a pro-inflammatory genomic signature. These results suggest that loss of Sirt2, as well as increased age, enhanced the immune response to pancreatic injury and induced an inflammatory phenotype permissive for the accumulation of cells carrying oncogenic Kras mutations.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
SIRT2 deletion enhances KRAS-induced tumorigenesis in vivo by regulating K147 acetylation status.Oncotarget. 2016 Dec 6;7(49):80336-80349. doi: 10.18632/oncotarget.12015. Oncotarget. 2016. PMID: 27637077 Free PMC article.
-
Tuft Cells Inhibit Pancreatic Tumorigenesis in Mice by Producing Prostaglandin D2.Gastroenterology. 2020 Nov;159(5):1866-1881.e8. doi: 10.1053/j.gastro.2020.07.037. Epub 2020 Jul 24. Gastroenterology. 2020. PMID: 32717220 Free PMC article.
-
NFATc1 Links EGFR Signaling to Induction of Sox9 Transcription and Acinar-Ductal Transdifferentiation in the Pancreas.Gastroenterology. 2015 May;148(5):1024-1034.e9. doi: 10.1053/j.gastro.2015.01.033. Epub 2015 Jan 23. Gastroenterology. 2015. PMID: 25623042 Free PMC article.
-
miR-802 Suppresses Acinar-to-Ductal Reprogramming During Early Pancreatitis and Pancreatic Carcinogenesis.Gastroenterology. 2022 Jan;162(1):269-284. doi: 10.1053/j.gastro.2021.09.029. Epub 2021 Sep 20. Gastroenterology. 2022. PMID: 34547282
-
Murine Models of Pancreatitis Leading to the Development of Pancreatic Cancer.Curr Protoc Pharmacol. 2018 Dec;83(1):e48. doi: 10.1002/cpph.48. Epub 2018 Oct 16. Curr Protoc Pharmacol. 2018. PMID: 30325112
Cited by
-
Metabolic mechanisms orchestrated by Sirtuin family to modulate inflammatory responses.Front Immunol. 2024 Sep 20;15:1448535. doi: 10.3389/fimmu.2024.1448535. eCollection 2024. Front Immunol. 2024. PMID: 39372420 Free PMC article. Review.
-
Sirtuins as Key Regulators in Pancreatic Cancer: Insights into Signaling Mechanisms and Therapeutic Implications.Cancers (Basel). 2024 Dec 6;16(23):4095. doi: 10.3390/cancers16234095. Cancers (Basel). 2024. PMID: 39682281 Free PMC article. Review.
-
L-arginine-induced pancreatitis aggravated by inhibiting Na+/Ca2+ exchanger 1.J Vet Med Sci. 2023 Jun 13;85(6):657-666. doi: 10.1292/jvms.22-0569. Epub 2023 May 23. J Vet Med Sci. 2023. PMID: 37100607 Free PMC article.
-
Emerging Potential Mechanism and Therapeutic Target of Ferroptosis in PDAC: A Promising Future.Int J Mol Sci. 2022 Nov 30;23(23):15031. doi: 10.3390/ijms232315031. Int J Mol Sci. 2022. PMID: 36499358 Free PMC article. Review.
-
SIRT2-mediated deacetylation and deubiquitination of C/EBPβ prevents ethanol-induced liver injury.Cell Discov. 2021 Oct 12;7(1):93. doi: 10.1038/s41421-021-00326-6. Cell Discov. 2021. PMID: 34642310 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

