Scaffold-Hopping from Synthetic Drugs by Holistic Molecular Representation
- PMID: 30405170
- PMCID: PMC6220272
- DOI: 10.1038/s41598-018-34677-0
Scaffold-Hopping from Synthetic Drugs by Holistic Molecular Representation
Abstract
The discovery of novel ligand chemotypes allows to explore uncharted regions in chemical space, thereby potentially improving synthetic accessibility, potency, and the drug-likeness of molecules. Here, we demonstrate the scaffold-hopping ability of the new Weighted Holistic Atom Localization and Entity Shape (WHALES) molecular descriptors compared to seven state-of-the-art molecular representations on 30,000 compounds and 182 biological targets. In a prospective application, we apply WHALES to the discovery of novel retinoid X receptor (RXR) modulators. WHALES descriptors identified four agonists with innovative molecular scaffolds, populating uncharted regions of the chemical space. One of the agonists, possessing a rare non-acidic chemotype, revealed high selectivity on 12 nuclear receptors and comparable efficacy as bexarotene on induction of ATP-binding cassette transporter A1, angiopoietin like protein 4 and apolipoprotein E. The outcome of this research supports WHALES as an innovative tool to explore novel regions of the chemical space and to detect novel bioactive chemotypes by straightforward similarity searching.
Conflict of interest statement
G.S. declares a potential financial conflict of interest in his role as life science industry consultant and cofounder of inSili.com GmbH, Zurich.
Figures

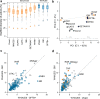


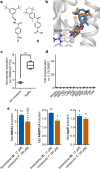
References
-
- Schneider G, Schneider P, Renner S. Scaffold-hopping: how far can you jump? Mol. Inf. 2006;25:1162–1171.
-
- Todeschini, R. & Consonni, V. Molecular Descriptors for Chemoinformatics41 (Wiley VCH, 2009).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

