Closed-type pre-treatment device for point-of-care testing of sputum
- PMID: 30405199
- PMCID: PMC6220321
- DOI: 10.1038/s41598-018-34781-1
Closed-type pre-treatment device for point-of-care testing of sputum
Erratum in
-
Author Correction: Closed-type pre-treatment device for point-of-care testing of sputum.Sci Rep. 2018 Dec 17;8(1):17994. doi: 10.1038/s41598-018-36359-3. Sci Rep. 2018. PMID: 30559366 Free PMC article.
Abstract
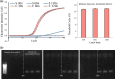
The procedures and protocols for the pre-treatment of sputum specimens, mainly used for the diagnosis of pneumonia, are complex, labor intensive, and require skilled specialists working in a biosafety containment laboratory because of sample infectivity. In this study, we developed the first portable, low-power pre-treatment device that carries out all sputum pre-treatment procedures (liquefaction, homogenization, dissolution, and inactivation) in an enclosed space. Designed to simultaneously employ chemical and mechanical dissolution in the enclosed chamber, this device eliminates the risk of transmission and improves the effectiveness of sputum dissolution and pathogen detection. This device is expected to allow for the pre-treatment of infectious sputum specimens outside of a biosafety containment laboratory. Used in conjunction with automated genome extraction and detection systems, this device should make the on-site diagnosis using infectious sputum specimens possible.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources

