Production of Cold-Active Lipase by Free and Immobilized Marine Bacillus cereus HSS: Application in Wastewater Treatment
- PMID: 30405541
- PMCID: PMC6205956
- DOI: 10.3389/fmicb.2018.02377
Production of Cold-Active Lipase by Free and Immobilized Marine Bacillus cereus HSS: Application in Wastewater Treatment
Abstract
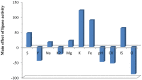
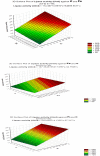
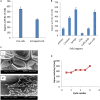
Lipases are enzymes that have the potential to hydrolyze triacylglycerol to free fatty acids and glycerol and have various applications. The aim of the present study was to isolate and screen marine bacteria for lipase production, optimize the production, and treat wastewater. A total of 20 marine bacterial isolates were obtained from the Mediterranean Sea and were screened for lipase production. All isolates were found to have lipolytic ability. The differences between the isolates were studied using RAPD-PCR. The most promising lipase producer (isolate 3) that exhibited the highest lipolytic hydrolysis (20 mm) was identified as Bacillus cereus HSS using 16S rDNA analysis and had the accession number MF581790. Optimization of lipase production was carried out using the Plackett-Burman experimental design with cotton seed oil as the inducer under shaking conditions at 10°C. The most significant factors that affected lipase production were FeSO4, KCl, and oil concentrations. By using the optimized culture conditions, the lipase activity increased by 1.8-fold compared with basal conditions. Immobilization by adsorption of cells on sponge and recycling raised lipase activity by 2.8-fold compared with free cells. The repeated reuse of the immobilized B. cereus HSS maintained reasonable lipase activity. A trial for the economic treatment of oily wastewater was carried out. Removal efficiencies of biological oxygen demand, total suspended solids, and oil and grease were 87.63, 90, and 94.7%, respectively, which is promising for future applications.
Keywords: Plackett–Burman; immobilization; lipase; recycling; wastewater treatment.
Figures






Similar articles
-
Optimization of the production conditions of the lipase produced by Bacillus cereus from rice flour through Plackett-Burman Design (PBD) and response surface methodology (RSM).Microb Pathog. 2016 Dec;101:36-43. doi: 10.1016/j.micpath.2016.10.020. Epub 2016 Nov 2. Microb Pathog. 2016. PMID: 27816679
-
Optimization of lipase production using fungal isolates from oily residues.BMC Biotechnol. 2021 Nov 10;21(1):65. doi: 10.1186/s12896-021-00724-4. BMC Biotechnol. 2021. PMID: 34758800 Free PMC article.
-
Lipolytic bacterial strains mediated transesterification of non-edible plant oils for generation of high quality biodiesel.J Biosci Bioeng. 2019 May;127(5):609-617. doi: 10.1016/j.jbiosc.2018.11.001. Epub 2018 Dec 19. J Biosci Bioeng. 2019. PMID: 30579829
-
Optimization of Lipase production from a novel strain Thalassospira permensis M35-15 using Response Surface Methodology.Bioengineered. 2016 Sep 2;7(5):298-303. doi: 10.1080/21655979.2016.1197713. Epub 2016 Jun 10. Bioengineered. 2016. PMID: 27285376 Free PMC article.
-
Upflow anaerobic sludge blanket reactor--a review.Indian J Environ Health. 2001 Apr;43(2):1-82. Indian J Environ Health. 2001. PMID: 12397675 Review.
Cited by
-
Homoscleromorpha-derived Bacillus spp. as potential sources of biotechnologically-relevant hydrolases and biosurfactants.World J Microbiol Biotechnol. 2022 Jul 27;38(10):169. doi: 10.1007/s11274-022-03358-6. World J Microbiol Biotechnol. 2022. PMID: 35882683
-
Harnessing the sponge microbiome for industrial biocatalysts.Appl Microbiol Biotechnol. 2020 Oct;104(19):8131-8154. doi: 10.1007/s00253-020-10817-3. Epub 2020 Aug 22. Appl Microbiol Biotechnol. 2020. PMID: 32827049 Review.
-
Metagenomic applications in exploration and development of novel enzymes from nature: a review.J Genet Eng Biotechnol. 2020 Aug 4;18(1):39. doi: 10.1186/s43141-020-00043-9. J Genet Eng Biotechnol. 2020. PMID: 32749574 Free PMC article. Review.
-
The Role of Plant Growth Promoting Rhizosphere Microbiome as Alternative Biofertilizer in Boosting Solanum melongena L. Adaptation to Salinity Stress.Plants (Basel). 2022 Feb 28;11(5):659. doi: 10.3390/plants11050659. Plants (Basel). 2022. PMID: 35270129 Free PMC article.
-
Characterization of Two Unique Cold-Active Lipases Derived from a Novel Deep-Sea Cold Seep Bacterium.Microorganisms. 2021 Apr 10;9(4):802. doi: 10.3390/microorganisms9040802. Microorganisms. 2021. PMID: 33920298 Free PMC article.
References
-
- Abd-Elnaby H., Beltagy E. A., Abo-Elela G. M., El-Sersy N. A. (2015). Achromobacter sp. and Virgibacillus pantothenticus as models of thermo-tolerant lipase-producing marine bacteria from North Delta sediments (Egypt). Afr. J. Microbiol. Res. 9 1001–1011. 10.5897/AJMR2014.6828 - DOI
-
- Anbu P., Noh M., Kim D., Seo J., Hur B., Min K. H. (2011). Screening and optimization of extracellular lipases by Acinetobacter species isolated from oil-contaminated soil in South Korea. Afr. J. Biotechnol. 10 4147–4156.
-
- Awad G., Mostafa H., Danial E. N., Abdelwahed N. A., Awad H. M. (2015). Enhanced production of thermostable lipase from Bacillus cereus ASSCRC-P1 in waste frying oil based medium using statistical experimental design. J. Appl. Pharm. Sci. 5 7–15. 10.7324/JAPS.2015.50902 - DOI
-
- Bala J. D., Lalung J., Ismail N. (2014). Biodegradation of palm oil mill effluent (POME) by bacteria. Int. J. Sci. Res. 4 1–10.
LinkOut - more resources
Full Text Sources

