Effects of 5-Azacytidine on Growth and Hypocrellin Production of Shiraia bambusicola
- PMID: 30405568
- PMCID: PMC6200910
- DOI: 10.3389/fmicb.2018.02508
Effects of 5-Azacytidine on Growth and Hypocrellin Production of Shiraia bambusicola
Abstract
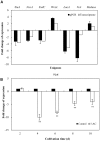
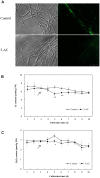
Hypocrellins, fungal perylenequinones of Shiraia bambusicola are developed as important photodynamic therapy agents against cancers and viruses. Due to the limitation of the wild resources, the mycelium culture is a promising alternative for hypocrellin production. As DNA methylation has profound effects on fungal growth, development and secondary metabolism, we used both McrBC cleavage and HPLC analysis to reveal the status of DNA methylation of S. bambusicola mycelium. We found that DNA methylation is absent in mycelia, but DNA methylation inhibitor 5-azacytidine (5-AC) still induced the fluffy phenotype and decreased hypocrellin contents significantly. Simultaneously, a total of 4,046 differentially expressed genes were induced by 5-AC, including up-regulated 2,392 unigenes (59.12%) and down-regulated 1,654 unigenes (40.88%). Gene ontology analysis showed 5-AC treatment changed expression of genes involved in membrane composition and oxidation-reduction process. The fluffy phenotype in 5-AC-treated S. bambusicola was closely related to strong promotion of developmental regulator WetA and the repression of the sexual developmental actor VeA and LaeA. It was a surprise finding that 5-AC reduced reactive oxygen species (ROS) production significantly in the mycelia via the inhibition of NADPH oxidase gene (NOX) expression and NOX activity. With the treatment of vitamin C and H2O2, we found that the reduced ROS generation was involved in the down-regulated expression of key genes for hypocrellin biosynthesis and the decreased hypocrellin production. To our knowledge, this is the first attempt to examine DNA methylation level in S. bambusicola. Our results suggested that the mediation of ROS generation could not be ignored in the study using 5-AC as a specific DNA methylation inhibitor.
Keywords: 5-azacytidine; DNA methylation; Shiraia bambusicola; fluffy; hypocrellin; reactive oxygen species; transcriptome analysis.
Figures






References
LinkOut - more resources
Full Text Sources
Research Materials

