Characterization of Diversity and Probiotic Efficiency of the Autochthonous Lactic Acid Bacteria in the Fermentation of Selected Raw Fruit and Vegetable Juices
- PMID: 30405588
- PMCID: PMC6205992
- DOI: 10.3389/fmicb.2018.02539
Characterization of Diversity and Probiotic Efficiency of the Autochthonous Lactic Acid Bacteria in the Fermentation of Selected Raw Fruit and Vegetable Juices
Abstract
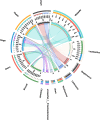
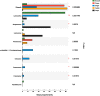
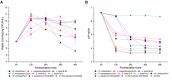
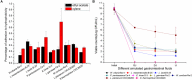
The diversity of indigenous lactic acid bacteria (LAB) in fermented broccoli, cherry, ginger, white radish, and white-fleshed pitaya juices was analyzed using culture-independent and -dependent approaches. The major properties of selected probiotic strains, including dynamic variations in pH, viable cell counts, antibiotic resistance, bacterial adhesion to hydrophobic compounds, and survivability during simulated gastrointestinal transit, were investigated using broccoli as the fermentation substrate. In broccoli and ginger juices, the genus Lactobacillus occupied the dominant position (abundances of 79.0 and 30.3%, respectively); in cherry and radish juices, Weissella occupied the dominant position (abundances of 78.3 and 83.2%, respectively); and in pitaya juice, Streptococcus and Lactococcus occupied the dominant positions (52.2 and 37.0%, respectively). Leuconostoc mesenteroides, Weissella cibaria/soli/confusa, Enterococcus gallinarum/durans/hirae, Pediococcus pentosaceus, Bacillus coagulans, and Lactococcus garvieae/lactis subspecies were identified by partial 16S rRNA gene sequencing. In general, the selected autochthonous LAB isolates displayed no significant differences in comparison with commercial strains with regard to growth rates or acidification in fermented broccoli juice. Among all the isolates, L. mesenteroides B4-25 exhibited the highest antibiotic resistance profile (equal to that of L. plantarum CICC20265), and suitable adhesion properties (adhesion of 13.4 ± 5.2% ∼ 36.4 ± 3.2% and 21.6 ± 1.4% ∼ 69.6 ± 2.3% to ethyl acetate and xylene, respectively). Furthermore, P. pentosaceus Ca-4 and L. mesenteroides B-25 featured the highest survival rates (22.4 ± 2.6 and 21.2 ± 1.4%, respectively), after simulated gastrointestinal transit. These results indicated a high level of diversity among the autochthonous bacterial community in fermented fruit and vegetable juices, and demonstrated the potential of these candidate probiotics for applications in fermentation.
Keywords: autochthonous lactic acid bacteria; fermentation; fruit and vegetable juice; microbial diversity; probiotic viability.
Figures

Similar articles
-
Pyrosequencing vs. culture-dependent approaches to analyze lactic acid bacteria associated to chicha, a traditional maize-based fermented beverage from Northwestern Argentina.Int J Food Microbiol. 2015 Apr 2;198:9-18. doi: 10.1016/j.ijfoodmicro.2014.12.027. Epub 2014 Dec 27. Int J Food Microbiol. 2015. PMID: 25584777
-
Molecular analysis of the dominant lactic acid bacteria of chickpea liquid starters and doughs and propagation of chickpea sourdoughs with selected Weissella confusa.Food Microbiol. 2020 Oct;91:103490. doi: 10.1016/j.fm.2020.103490. Epub 2020 Apr 1. Food Microbiol. 2020. PMID: 32539978
-
Composition of lactic acid bacteria during spontaneous curly kale (Brassica oleracea var. sabellica) fermentation.Microbiol Res. 2018 Jan;206:121-130. doi: 10.1016/j.micres.2017.09.011. Epub 2017 Oct 16. Microbiol Res. 2018. PMID: 29146249
-
Selection and use of autochthonous mixed starter for lactic acid fermentation of carrots, French beans or marrows.Int J Food Microbiol. 2008 Oct 31;127(3):220-8. doi: 10.1016/j.ijfoodmicro.2008.07.010. Epub 2008 Jul 13. Int J Food Microbiol. 2008. PMID: 18710789
-
The consequences of fermentation metabolism on the qualitative qualities and biological activity of fermented fruit and vegetable juices.Food Chem X. 2024 Feb 10;21:101209. doi: 10.1016/j.fochx.2024.101209. eCollection 2024 Mar 30. Food Chem X. 2024. PMID: 38384684 Free PMC article. Review.
Cited by
-
The indigenous microbial diversity involved in the spontaneous fermentation of red dragon fruit (Hylocereus polyrhizus) identified by means of molecular tools.Heliyon. 2023 Nov 7;9(11):e21940. doi: 10.1016/j.heliyon.2023.e21940. eCollection 2023 Nov. Heliyon. 2023. PMID: 38027851 Free PMC article.
-
Use of Autochthonous Lactobacilli to Increase the Safety of Zgougou.Microorganisms. 2019 Dec 22;8(1):29. doi: 10.3390/microorganisms8010029. Microorganisms. 2019. PMID: 31877880 Free PMC article.
-
In Vitro Probiotic Properties and In Vivo Anti-Ageing Effects of Lactoplantibacillus plantarum PFA2018AU Strain Isolated from Carrots on Caenorhabditis elegans.Microorganisms. 2023 Apr 21;11(4):1087. doi: 10.3390/microorganisms11041087. Microorganisms. 2023. PMID: 37110510 Free PMC article.
-
Microbial communities of a variety of 75 homemade fermented vegetables.Front Microbiol. 2023 Dec 15;14:1323424. doi: 10.3389/fmicb.2023.1323424. eCollection 2023. Front Microbiol. 2023. PMID: 38163080 Free PMC article.
-
Recent Innovations in Non-dairy Prebiotics and Probiotics: Physiological Potential, Applications, and Characterization.Probiotics Antimicrob Proteins. 2023 Apr;15(2):239-263. doi: 10.1007/s12602-022-09983-9. Epub 2022 Sep 5. Probiotics Antimicrob Proteins. 2023. PMID: 36063353 Review.
References
-
- Adewumi G. A., Oguntoyinbo F. A., Keisam S., Romi W., Jeyaram K. (2013). Combination of culture-independent and culture-dependent molecular methods for the determination of bacterial community of iru, a fermented Parkia biglobosa seeds. Front. Microbiol. 3:436. 10.3389/Fmicb.2012.00436 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

