Identification of wysPII as an Activator of Morphological Development in Streptomyces albulus CK-15
- PMID: 30405594
- PMCID: PMC6207912
- DOI: 10.3389/fmicb.2018.02550
Identification of wysPII as an Activator of Morphological Development in Streptomyces albulus CK-15
Abstract
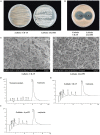
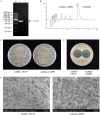
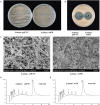
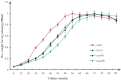
Wuyiencin is produced by Streptomyces albulus var. wuyiensis and used widely in agriculture to control a variety of fungal diseases, such as cucumber downy mildew, strawberry powdery mildew, and tomato gray mold. As an industrially-produced biopesticide, reducing production costs is very important for popularization of this approach. To obtain a rapidly growing strain that effectively shortens the fermentation time, we investigated the effects of knockout and overexpression of the wysPII gene, a member of the LuxR regulatory gene family, in S. albulus strain CK-15. The ΔwysPII mutant exhibited a reduced rate of growth and sporulation. The time taken to reach the greatest mycelial biomass was approximately 18 h shorter in the ooPII (wysPII overexpressing) strain compared with that of the wild-type (WT) strain. In addition, the time to reach the greatest wuyiencin production was 56 h in the ooPII strain compared with 62 h in the WT strain. Furthermore, wysPII was shown to act as an activator of morphological development without affecting wuyiencin production. Thus, the ooPII strain can be used to reduce costs and increase efficiency in industrial fermentation processes for wuyiencin production.
Keywords: LuxR family; Streptomyces albulus; morphological development; regulatory genes; wysPII.
Figures





Similar articles
-
Effect of toyF on wuyiencin and toyocamycin production by Streptomyces albulus CK-15.World J Microbiol Biotechnol. 2022 Mar 1;38(4):65. doi: 10.1007/s11274-022-03234-3. World J Microbiol Biotechnol. 2022. PMID: 35229201
-
Overexpression of wysR gene enhances wuyiencin production in ΔwysR3 mutant strain of Streptomyces albulus var. wuyiensis strain CK-15.J Appl Microbiol. 2020 Sep;129(3):565-574. doi: 10.1111/jam.14629. Epub 2020 Apr 6. J Appl Microbiol. 2020. PMID: 32145135
-
Wuyiencin produced by Streptomyces albulus CK-15 displays biocontrol activities against cucumber powdery mildew.J Appl Microbiol. 2021 Dec;131(6):2957-2970. doi: 10.1111/jam.15168. Epub 2021 Jun 26. J Appl Microbiol. 2021. PMID: 34060684
-
Characterization of novel DeoR-family member from the Streptomyces ahygroscopicus strain CK-15 that acts as a repressor of morphological development.Appl Microbiol Biotechnol. 2016 Oct;100(20):8819-28. doi: 10.1007/s00253-016-7661-y. Epub 2016 Jul 2. Appl Microbiol Biotechnol. 2016. PMID: 27372076
-
Improvement of Wuyiencin biosynthesis in Streptomyces wuyiensis CK-15 by identification of a key regulator, WysR.J Microbiol Biotechnol. 2014 Dec 28;24(12):1644-53. doi: 10.4014/jmb.1405.05017. J Microbiol Biotechnol. 2014. PMID: 25112317
Cited by
-
Effect of toyF on wuyiencin and toyocamycin production by Streptomyces albulus CK-15.World J Microbiol Biotechnol. 2022 Mar 1;38(4):65. doi: 10.1007/s11274-022-03234-3. World J Microbiol Biotechnol. 2022. PMID: 35229201
-
iTRAQ-based proteomic analysis reveals the mechanisms of Botrytis cinerea controlled with Wuyiencin.BMC Microbiol. 2019 Dec 11;19(1):280. doi: 10.1186/s12866-019-1675-4. BMC Microbiol. 2019. PMID: 31829181 Free PMC article.
-
Comprehensive genome analysis of Lentzea reveals repertoire of polymer-degrading enzymes and bioactive compounds with clinical relevance.Sci Rep. 2022 May 19;12(1):8409. doi: 10.1038/s41598-022-12427-7. Sci Rep. 2022. PMID: 35589875 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials

