Interpretation of Serological Complement Biomarkers in Disease
- PMID: 30405598
- PMCID: PMC6207586
- DOI: 10.3389/fimmu.2018.02237
Interpretation of Serological Complement Biomarkers in Disease
Abstract
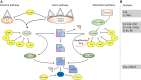
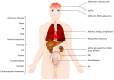
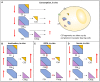
Complement system aberrations have been identified as pathophysiological mechanisms in a number of diseases and pathological conditions either directly or indirectly. Examples of such conditions include infections, inflammation, autoimmune disease, as well as allogeneic and xenogenic transplantation. Both prospective and retrospective studies have demonstrated significant complement-related differences between patient groups and controls. However, due to the low degree of specificity and sensitivity of some of the assays used, it is not always possible to make predictions regarding the complement status of individual patients. Today, there are three main indications for determination of a patient's complement status: (1) complement deficiencies (acquired or inherited); (2) disorders with aberrant complement activation; and (3) C1 inhibitor deficiencies (acquired or inherited). An additional indication is to monitor patients on complement-regulating drugs, an indication which may be expected to increase in the near future since there is now a number of such drugs either under development, already in clinical trials or in clinical use. Available techniques to study complement include quantification of: (1) individual components; (2) activation products, (3) function, and (4) autoantibodies to complement proteins. In this review, we summarize the appropriate indications, techniques, and interpretations of basic serological complement analyses, exemplified by a number of clinical disorders.
Keywords: activation products; complement; complement regulatory drugs; deficiency; functional test.
Figures
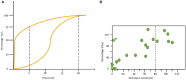
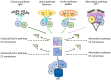
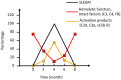
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

