Incubation of Immune Cell Grafts With MAX.16H5 IgG1 Anti-Human CD4 Antibody Prolonged Survival After Hematopoietic Stem Cell Transplantation in a Mouse Model for Fms Like Tyrosine Kinase 3 Positive Acute Myeloid Leukemia
- PMID: 30405611
- PMCID: PMC6204383
- DOI: 10.3389/fimmu.2018.02408
Incubation of Immune Cell Grafts With MAX.16H5 IgG1 Anti-Human CD4 Antibody Prolonged Survival After Hematopoietic Stem Cell Transplantation in a Mouse Model for Fms Like Tyrosine Kinase 3 Positive Acute Myeloid Leukemia
Abstract
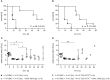
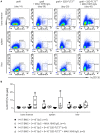
Despite the constant development of innovative therapeutic options for hematological malignancies, the gold-standard therapy regimen for curative treatment often includes allogeneic hematopoietic stem cell transplantation (HSCT). The graft-vs.-leukemia effect (GVL) is one of the main therapeutic goals that arises from HSCT. On the other hand, graft-vs.-host disease (GVHD) is still one of the main and most serious complications following allogeneic HSCT. In acute myeloid leukemia (AML), HSCT together with high-dose chemotherapy is used as a treatment option. An aggressive progression of the disease, a decreased response to treatment, and a poor prognosis are connected to internal tandem duplication (ITD) mutations in the Fms like tyrosine kinase 3 (FLT3) gene, which affects around 30% of AML patients. In this study, C3H/HeN mice received an allogeneic graft together with 32D-FLT3ITD AML cells to induce acute GVHD and GVL. It was examined if pre-incubation of the graft with the anti-human cluster of differentiation (CD) 4 antibody MAX.16H5 IgG1 prevented the development of GVHD and whether the graft function was impaired. Animals receiving grafts pre-incubated with the antibody together with FLT3ITD AML cells survived significantly longer than mice receiving untreated grafts. The observed prolonged survival due to MAX.16H5 incubation of immune cell grafts prior to transplantation may allow an extended application of additional targeted strategies in the treatment of AML.
Keywords: 32D-FLT3ITD; C3H/HeN; acute myeloid leukemia; anti-human CD4 antibody MAX.16H5; graft-vs.-host disease; graft-vs.-leukemia; hematopoietic stem cell transplantation.
Figures



Similar articles
-
The Epitope-Specific Anti-human CD4 Antibody MAX.16H5 and Its Role in Immune Tolerance.Front Immunol. 2019 May 24;10:1035. doi: 10.3389/fimmu.2019.01035. eCollection 2019. Front Immunol. 2019. PMID: 31178857 Free PMC article. Review.
-
Mild chronic graft-versus-host disease may alleviate poor prognosis associated with FLT3 internal tandem duplication for adult acute myeloid leukemia following allogeneic stem cell transplantation with myeloablative conditioning in first complete remission: a retrospective study.Eur J Haematol. 2016 Mar;96(3):236-44. doi: 10.1111/ejh.12575. Epub 2015 May 18. Eur J Haematol. 2016. PMID: 25912052
-
Flow cytometric analysis of the graft-versus-Leukemia-effect after hematopoietic stem cell transplantation in mice.Cytometry A. 2015 Apr;87(4):334-45. doi: 10.1002/cyto.a.22619. Epub 2015 Feb 24. Cytometry A. 2015. PMID: 25717029
-
Phase I trial of maintenance sorafenib after allogeneic hematopoietic stem cell transplantation for fms-like tyrosine kinase 3 internal tandem duplication acute myeloid leukemia.Biol Blood Marrow Transplant. 2014 Dec;20(12):2042-8. doi: 10.1016/j.bbmt.2014.09.007. Epub 2014 Sep 17. Biol Blood Marrow Transplant. 2014. PMID: 25239228 Free PMC article. Clinical Trial.
-
Allogeneic Hematopoietic Stem Cell Transplantation in FLT3-ITD-Positive Acute Myelogenous Leukemia: The Role for FLT3 Tyrosine Kinase Inhibitors Post-Transplantation.Biol Blood Marrow Transplant. 2016 Jun;22(6):982-990. doi: 10.1016/j.bbmt.2016.01.013. Epub 2016 Jan 16. Biol Blood Marrow Transplant. 2016. PMID: 26785334 Review.
Cited by
-
The Epitope-Specific Anti-human CD4 Antibody MAX.16H5 and Its Role in Immune Tolerance.Front Immunol. 2019 May 24;10:1035. doi: 10.3389/fimmu.2019.01035. eCollection 2019. Front Immunol. 2019. PMID: 31178857 Free PMC article. Review.
-
Humanized mouse model: Hematopoietic stemcell transplantation and tracking using short tandem repeat technology.Immun Inflamm Dis. 2020 Sep;8(3):363-370. doi: 10.1002/iid3.317. Epub 2020 Jun 11. Immun Inflamm Dis. 2020. PMID: 32525618 Free PMC article.
-
Hematopoietic stem cells: Understanding the mechanisms to unleash the therapeutic potential of hematopoietic stem cell transplantation.Stem Cell Res Ther. 2025 Feb 10;16(1):60. doi: 10.1186/s13287-024-04126-z. Stem Cell Res Ther. 2025. PMID: 39924510 Free PMC article. Review.
References
-
- Ottinger HD, Ferencik S, Beelen DW, Lindemann M, Peceny R, Elmaagacli AH, et al. . Hematopoietic stem cell transplantation: contrasting the outcome of transplantations from HLA-identical siblings, partially HLA-mismatched related donors, and HLA-matched unrelated donors. Blood (2003) 102:1131–7. 10.1182/blood-2002-09-2866 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

