Role of TRAFs in Signaling Pathways Controlling T Follicular Helper Cell Differentiation and T Cell-Dependent Antibody Responses
- PMID: 30405612
- PMCID: PMC6204373
- DOI: 10.3389/fimmu.2018.02412
Role of TRAFs in Signaling Pathways Controlling T Follicular Helper Cell Differentiation and T Cell-Dependent Antibody Responses
Abstract
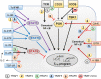
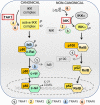
Follicular helper T (TFH) cells represent a highly specialized CD4+ T cell subpopulation that supports the generation of germinal centers (GC) and provides B cells with critical signals promoting antibody class switching, generation of high affinity antibodies, and memory formation. TFH cells are characterized by the expression of the chemokine receptor CXCR5, the transcription factor Bcl-6, costimulatory molecules ICOS, and PD-1, and the production of cytokine IL-21. The acquisition of a TFH phenotype is a complex and multistep process that involves signals received through engagement of the TCR along with a multitude of costimulatory molecules and cytokines receptors. Members of the Tumor necrosis factor Receptor Associated Factors (TRAF) represent one of the major classes of signaling mediators involved in the differentiation and functions of TFH cells. TRAF molecules are the canonical adaptor molecules that physically interact with members of the Tumor Necrosis Factor Receptor Superfamily (TNFRSF) and actively modulate their downstream signaling cascades through their adaptor function and/or E3 ubiquitin ligase activity. OX-40, GITR, and 4-1BB are the TRAF-dependent TNFRSF members that have been implicated in the differentiation and functions of TFH cells. On the other hand, emerging data demonstrate that TRAF proteins also participate in signaling from the TCR and CD28, which deliver critical signals leading to the differentiation of TFH cells. More intriguingly, we recently showed that the cytoplasmic tail of ICOS contains a conserved TANK-binding kinase 1 (TBK1)-binding motif that is shared with TBK1-binding TRAF proteins. The presence of this TRAF-mimicking signaling module downstream of ICOS is required to mediate the maturation step during TFH differentiation. In addition, JAK-STAT pathways emanating from IL-2, IL-6, IL-21, and IL-27 cytokine receptors affect TFH development, and crosstalk between TRAF-mediated pathways and the JAK-STAT pathways can contribute to generate integrated signals required to drive and sustain TFH differentiation. In this review, we will introduce the molecular interactions and the major signaling pathways controlling the differentiation of TFH cells. In each case, we will highlight the contributions of TRAF proteins to these signaling pathways. Finally, we will discuss the role of individual TRAF proteins in the regulation of T cell-dependent humoral responses.
Keywords: NF-κB; TCR signaling; TRAF; antibody response; costimulation signaling; cytokine signaling; follicular helper T cell.
Figures
Similar articles
-
CD25(+) Bcl6(low) T follicular helper cells provide help to maturing B cells in germinal centers of human tonsil.Eur J Immunol. 2015 Jan;45(1):298-308. doi: 10.1002/eji.201444911. Epub 2014 Oct 27. Eur J Immunol. 2015. PMID: 25263533 Free PMC article.
-
Signals that influence T follicular helper cell differentiation and function.Semin Immunopathol. 2010 Jun;32(2):183-96. doi: 10.1007/s00281-009-0194-z. Epub 2010 Jan 27. Semin Immunopathol. 2010. PMID: 20107805 Review.
-
The transforming growth factor beta signaling pathway is critical for the formation of CD4 T follicular helper cells and isotype-switched antibody responses in the lung mucosa.Elife. 2015 Jan 8;4:e04851. doi: 10.7554/eLife.04851. Elife. 2015. PMID: 25569154 Free PMC article.
-
Signal Transducer and Activator of Transcription 3 Hyperactivation Associates With Follicular Helper T Cell Differentiation and Disease Activity in Rheumatoid Arthritis.Front Immunol. 2018 Jun 4;9:1226. doi: 10.3389/fimmu.2018.01226. eCollection 2018. Front Immunol. 2018. PMID: 29915585 Free PMC article.
-
Transcriptional and epigenetic regulation of follicular T-helper cells and their role in autoimmunity.Autoimmunity. 2017 Mar;50(2):71-81. doi: 10.1080/08916934.2017.1284821. Epub 2017 Feb 21. Autoimmunity. 2017. PMID: 28263097 Review.
Cited by
-
Follicular Helper T Cells in the Immunopathogenesis of SARS-CoV-2 Infection.Front Immunol. 2021 Sep 16;12:731100. doi: 10.3389/fimmu.2021.731100. eCollection 2021. Front Immunol. 2021. PMID: 34603308 Free PMC article. Review.
-
A quinazoline derivative suppresses B cell hyper-activation and ameliorates the severity of systemic lupus erythematosus in mice.Front Pharmacol. 2023 May 15;14:1159075. doi: 10.3389/fphar.2023.1159075. eCollection 2023. Front Pharmacol. 2023. PMID: 37256224 Free PMC article.
-
NF-κB over-activation portends improved outcomes in HPV-associated head and neck cancer.Oncotarget. 2022 May 24;13:707-722. doi: 10.18632/oncotarget.28232. eCollection 2022. Oncotarget. 2022. PMID: 35634245 Free PMC article.
-
Immunoprofiling of 4-1BB Expression Predicts Outcome in Chronic Lymphocytic Leukemia (CLL).Diagnostics (Basel). 2021 Nov 4;11(11):2041. doi: 10.3390/diagnostics11112041. Diagnostics (Basel). 2021. PMID: 34829391 Free PMC article.
-
Total Alkaloids of Sophora alopecuroides Linn. Attenuates Rheumatoid Arthritis Through Regulating Follicular Helper T Cells.J Inflamm Res. 2024 Jun 4;17:3587-3602. doi: 10.2147/JIR.S449330. eCollection 2024. J Inflamm Res. 2024. PMID: 38860009 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

