Invasive bronchial fibroblasts derived from asthmatic patients activate lung cancer A549 cells in vitro
- PMID: 30405798
- PMCID: PMC6202494
- DOI: 10.3892/ol.2018.9462
Invasive bronchial fibroblasts derived from asthmatic patients activate lung cancer A549 cells in vitro
Abstract
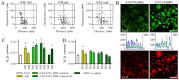
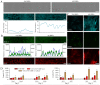
Epidemiological data suggests that there are functional links between bronchial asthma and lung carcinogenesis. Bronchial fibroblasts serve a prominent role in the asthmatic process; however, their involvement in lung cancer progression remains unaddressed. To estimate the effect of the asthmatic microenvironment on the invasiveness of lung cancer cells, the present study compared the behavior of human non-small cell lung cancer A549 cells exposed to the signals from human bronchial fibroblasts (HBFs) derived from non-asthmatic donors (NA HBFs) and from asthmatic patients (AS HBFs). NA HBFs did not significantly affect A549 motility, whereas AS HBFs and the media conditioned with AS HBF/A549 co-cultures increased Snail-1/connexin43 expression and motility of A549 cells. In contrast to NA HBFs, which formed A549-impenetrable lateral barriers, α-SMA+ AS HBFs actively infiltrated A549 monolayers and secreted chemotactic factors that arrested A549 cells within AS HBF/A549 contact zone. However, small sub-populations of A549 cells could release from this arrest and colonize distant regions of AS HBF monolayers. These data indicated that the interactions between lung cancer cells and HBFs in asthmatic bronchi may facilitate the colonization of lung tumors by fibroblasts. It further stabilizes the tumor microenvironment and potentially facilitates collective colonization of novel bronchial loci by cancer cells. Potential mechanistic links between the asthmatic process and lung cancer progression suggest that bronchial asthma should be included in the list of potential prognostic markers for lung cancer therapy.
Keywords: CAFs; bronchial asthma; intercellular communication; invasion; lung cancer.
Figures

Similar articles
-
Undifferentiated bronchial fibroblasts derived from asthmatic patients display higher elastic modulus than their non-asthmatic counterparts.PLoS One. 2015 Feb 13;10(2):e0116840. doi: 10.1371/journal.pone.0116840. eCollection 2015. PLoS One. 2015. PMID: 25679502 Free PMC article.
-
Asthmatic bronchial fibroblasts demonstrate enhanced potential to differentiate into myofibroblasts in culture.Med Sci Monit. 2009 Jul;15(7):BR194-201. Med Sci Monit. 2009. PMID: 19564819
-
Apigenin inhibits TGF-β1 induced fibroblast-to-myofibroblast transition in human lung fibroblast populations.Pharmacol Rep. 2013;65(1):164-72. doi: 10.1016/s1734-1140(13)70974-5. Pharmacol Rep. 2013. PMID: 23563034
-
Lithium Attenuates TGF-β(1)-Induced Fibroblasts to Myofibroblasts Transition in Bronchial Fibroblasts Derived from Asthmatic Patients.J Allergy (Cairo). 2012;2012:206109. doi: 10.1155/2012/206109. Epub 2012 Sep 3. J Allergy (Cairo). 2012. PMID: 22988467 Free PMC article.
-
IL-5 and IL-5 receptor in asthma.Mem Inst Oswaldo Cruz. 1997;92 Suppl 2:75-91. doi: 10.1590/s0074-02761997000800012. Mem Inst Oswaldo Cruz. 1997. PMID: 9698919 Review.
Cited by
-
Porous Polymeric Microspheres With Controllable Pore Diameters for Tissue Engineered Lung Tumor Model Development.Front Bioeng Biotechnol. 2020 Jul 10;8:799. doi: 10.3389/fbioe.2020.00799. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32754585 Free PMC article.
-
Scutellaria baicalensis and Their Natural Flavone Compounds as Potential Medicinal Drugs for the Treatment of Nicotine-Induced Non-Small-Cell Lung Cancer and Asthma.Int J Environ Res Public Health. 2021 May 14;18(10):5243. doi: 10.3390/ijerph18105243. Int J Environ Res Public Health. 2021. PMID: 34069141 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials
