Hyperthermia enhances the sensitivity of pancreatic cancer SW1990 cells to gemcitabine through ROS/JNK signaling
- PMID: 30405817
- PMCID: PMC6202550
- DOI: 10.3892/ol.2018.9455
Hyperthermia enhances the sensitivity of pancreatic cancer SW1990 cells to gemcitabine through ROS/JNK signaling
Abstract
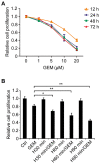
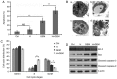
Pancreatic cancer (PC) is a highly aggressive type of cancer. Gemcitabine (GEM) is a standard chemotherapeutic treatment of advanced PC; however, it requires improvement, and more effective therapeutic methods must be further explored. In the present study, hyperthermia combined with GEM was used on the PC cell line SW1990. The results revealed that mild hyperthermia (at 42°C) effectively increased the inhibitory effect of GEM on cell viability, as determined using an MTT assay, and increased the effect of GEM-induced apoptosis, as determined using an Annexin V-fluorescein isothiocyanate/propidium iodide assay, in PC SW1990 cells. Additionally, it resulted in increased S-phase arrest, downregulated the expression of the anti-apoptosis protein B-cell lymphoma 2 and upregulated the expression of the pro-apoptosis protein Bcl-2-associated X protein, cleaved caspase-3 and cleaved caspase-9, as determined using a reverse transcription-quantitative polymerase chain reaction and western blot analysis. Furthermore, it was revealed that hyperthermia resulted in the rapid generation of reactive oxygen species (ROS) and substantial activation of c-Jun-N-terminal kinase (JNK). The introduction of ROS and JNK inhibitors suppressed hyperthermia-induced apoptosis in GEM-treated cells, suggesting that hyperthermia increased GEM cytotoxicity in PC SW1990 cells by inducing apoptosis via the ROS/JNK signaling pathway.
Keywords: apoptosis; gemcitabine; hyperthermia; pancreatic cancer; reactive oxygen species/c-Jun-N-terminal kinase signaling; viability.
Figures

References
-
- Worni M, Guller U, White RR, Castleberry AW, Pietrobon R, Cerny T, Gloor B, Koeberle D. Modest improvement in overall survival for patients with metastatic pancreatic cancer: A trend analysis using the surveillance, epidemiology, and end results registry from 1988 to 2008. Pancreas. 2013;42:1157–1163. doi: 10.1097/MPA.0b013e318291fbc5. - DOI - PubMed
-
- Ottaiano A, Capozzi M, De Divitiis C, De Stefano A, Botti G, Avallone A, Tafuto S. Gemcitabine mono-therapy versus gemcitabine plus targeted therapy in advanced pancreatic cancer: A meta-analysis of randomized phase III trials. Acta Oncol. 2017;56:377–383. doi: 10.1080/0284186X.2017.1288922. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
