Hsa-miR-376c-3p targets Cyclin D1 and induces G1-cell cycle arrest in neuroblastoma cells
- PMID: 30405823
- PMCID: PMC6202480
- DOI: 10.3892/ol.2018.9431
Hsa-miR-376c-3p targets Cyclin D1 and induces G1-cell cycle arrest in neuroblastoma cells
Abstract
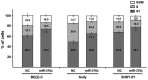
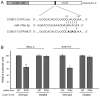
High-risk neuroblastoma is the most aggressive form of cancer in children. The estimated survival of children with high-risk neuroblastoma is 40-50% compared with low and intermediate risk neuroblastoma, which is >98 and 90-95%, respectively. In addition, patients with high-risk neuroblastoma often experience relapse following intensive treatments with standard chemotherapeutic drugs. Therefore alternative strategies are required to address this problem. MicroRNAs (miRNAs/miRs) are small, endogenously expressed non-coding RNAs, which when deregulated have been demonstrated to serve significant roles in the tumorigenesis of a number of different types of cancer. Results from a previous deep sequencing study identified 22 downregulated miRNAs from the 14q32 miRNA cluster differentially expressed in neuroblastoma cell lines isolated from 6 patients at diagnosis and at relapse following intensive treatments. miR-376c-3p is one of the 22 miRNAs that was downregulated in the majority of the cell lines isolated from patients post treatment. The present study employed reverse transcription-quantitative polymerase chain reaction (RT-qPCR) to quantify the basic expression of miR-376c-3p in 6 neuroblastoma cell lines. The functional role of miR-376c-3p in the neuroblastoma cell lines was evaluated by alamar blue-cell viability and propidium iodide-flow cytometric assays. In addition, luciferase reporter assays, RT-qPCR and western blotting were performed to identify and quantify the targets of miR-376c-3p in neuroblastoma cell lines. Ectopic expression of miR-376c-3p led to significant inhibition of cell viability and G1-cell cycle arrest in multiple neuroblastoma cell lines by reducing the expression of cyclin D1, an oncogene critical for neuroblastoma pathogenesis. The results of the present study provide novel insights into the functional role of miR-376c-3p and suggest new approaches for the treatment of neuroblastoma.
Keywords: 14q32 microRNA cluster; Cyclin D1; deep sequencing; high-risk neuroblastoma; microRNA; microRNA-376c-3p.
Figures




Similar articles
-
miR-376c-3p modulates the properties of breast cancer stem cells by targeting RAB2A.Exp Ther Med. 2020 Nov;20(5):68. doi: 10.3892/etm.2020.9196. Epub 2020 Sep 9. Exp Ther Med. 2020. PMID: 32963598 Free PMC article.
-
Hsa-miR-323a-3p functions as a tumor suppressor and targets STAT3 in neuroblastoma cells.Front Pediatr. 2023 Mar 24;11:1098999. doi: 10.3389/fped.2023.1098999. eCollection 2023. Front Pediatr. 2023. PMID: 37033189 Free PMC article.
-
Upregulation of miR-376c-3p alleviates oxygen-glucose deprivation-induced cell injury by targeting ING5.Cell Mol Biol Lett. 2019 Dec 4;24:67. doi: 10.1186/s11658-019-0189-2. eCollection 2019. Cell Mol Biol Lett. 2019. PMID: 31844418 Free PMC article.
-
MicroRNA-193a-3p inhibits cell proliferation in prostate cancer by targeting cyclin D1.Oncol Lett. 2017 Nov;14(5):5121-5128. doi: 10.3892/ol.2017.6865. Epub 2017 Sep 1. Oncol Lett. 2017. PMID: 29142597 Free PMC article.
-
A novel miR-0308-3p revealed by miRNA-seq of HBV-positive hepatocellular carcinoma suppresses cell proliferation and promotes G1/S arrest by targeting double CDK6/Cyclin D1 genes.Cell Biosci. 2020 Feb 27;10:24. doi: 10.1186/s13578-020-00382-7. eCollection 2020. Cell Biosci. 2020. PMID: 32128112 Free PMC article.
Cited by
-
ClustMMRA v2: A Scalable Computational Pipeline for the Identification of MicroRNA Clusters Acting Cooperatively on Tumor Molecular Subgroups.Adv Exp Med Biol. 2022;1385:259-279. doi: 10.1007/978-3-031-08356-3_10. Adv Exp Med Biol. 2022. PMID: 36352218
-
MCPIP1 modulates the miRNA‒mRNA landscape in keratinocyte carcinomas.J Exp Clin Cancer Res. 2024 Oct 21;43(1):290. doi: 10.1186/s13046-024-03211-8. J Exp Clin Cancer Res. 2024. PMID: 39428471 Free PMC article.
-
Deregulated expression of the 14q32 miRNA cluster in clear cell renal cancer cells.Front Oncol. 2023 Apr 17;13:1048419. doi: 10.3389/fonc.2023.1048419. eCollection 2023. Front Oncol. 2023. PMID: 37139155 Free PMC article.
-
Loading MicroRNA-376c in Extracellular Vesicles Inhibits Properties of Non-Small Cell Lung Cancer Cells by Targeting YTHDF1.Technol Cancer Res Treat. 2020 Jan-Dec;19:1533033820977525. doi: 10.1177/1533033820977525. Technol Cancer Res Treat. 2020. PMID: 33280517 Free PMC article.
-
miR-376c-3p modulates the properties of breast cancer stem cells by targeting RAB2A.Exp Ther Med. 2020 Nov;20(5):68. doi: 10.3892/etm.2020.9196. Epub 2020 Sep 9. Exp Ther Med. 2020. PMID: 32963598 Free PMC article.
References
-
- Garaventa A, Parodi S, De Bernardi B, Dau D, Manzitti C, Conte M, Casale F, Viscardi E, Bianchi M, D'Angelo P, et al. Outcome of children with neuroblastoma after progression or relapse. A retrospective study of the Italian neuroblastoma registry. Eur J Cancer. 2009;45:2835–2842. doi: 10.1016/j.ejca.2009.06.010. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
