Assessment of a mouse xenograft model of primary colorectal cancer with special reference to perfluorooctane sulfonate
- PMID: 30405966
- PMCID: PMC6216948
- DOI: 10.7717/peerj.5602
Assessment of a mouse xenograft model of primary colorectal cancer with special reference to perfluorooctane sulfonate
Abstract
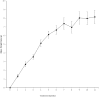
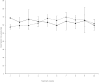
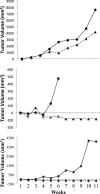
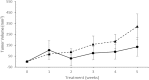
Colorectal cancer ranks third among the most commonly diagnosed cancers in the United States. Current therapies have a range of side effects, and the development of a reliable animal model to speed the discovery of safe effective preventative therapies would be of great value. A cross-sectional study in a large Appalachian population recently showed an association between low circulating levels of perfluorooctane sulfonate (PFOS) and a reduced prevalence of colorectal cancer. A study using APCmin (C57BL/6J-ApcMin/J) mice prone to familial adenomatous polyposis found PFOS was protective when exposure occurred during tumor development. To test the possible benefit of PFOS on spontaneous colorectal cancer, we developed a mouse model utilizing primary patient colorectal cancer implants into NSG (NOD.Cg-PrkdcscidIl2rgtm1Wjl /Sz) mice. Study goals included: (1) to assess potential factors supporting the successful use of colorectal cancer from heterogeneous tumors for PDX studies; and, (2) evaluate PFOS as a therapy in tumor matched pairs of mice randomized to receive PFOS or vehicle. The time in days for mice to grow primary tumors to 5 mm took almost 2 months (mean = 53.3, se = 5.7, range = 17-136). Age of mice at implantation, patient age, gender and race appeared to have no discernable effect on engraftment rates. Engraftment rates for low and high-grade patient tumors were similar. PFOS appeared to reduce tumor size dramatically in one group of tumors, those from the right ascending colon. That is, by 5 weeks of treatment in two mice, PFOS had eliminated their 52.4 mm3 and 124.6 mm3 masses completely, an effect that was sustained for 10 weeks of treatment; in contrast, their corresponding matched vehicle control mice had tumors that grew to 472.7 mm3 and 340.1 mm3 in size respectively during the same period. In a third xenograft mouse, the tumor growth was dramatically blunted although not eliminated, and compared favorably to their matched vehicle controls over the same period. These preliminary findings suggested that this mouse model may be advantageous for testing compounds of potential value in the treatment of colorectal cancer, and PFOS may have utility in selected cases.
Keywords: CRC; Model selection; NOD SCID Gamma mouse; NSG mouse; PDX; PFOS; Patient derived xenograft; Perfluorooctane sulfonate; Treatment.
Conflict of interest statement
The authors declare there are no competing interests.
Figures
Similar articles
-
Oral perfluorooctane sulfonate (PFOS) lessens tumor development in the APCmin mouse model of spontaneous familial adenomatous polyposis.BMC Cancer. 2016 Dec 8;16(1):942. doi: 10.1186/s12885-016-2861-5. BMC Cancer. 2016. PMID: 27927180 Free PMC article.
-
Degenerative Myelopathy and Neuropathy in NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) Mice Caused by Lactate Dehydrogenase-Elevating Virus (LDV).Toxicol Pathol. 2022 Apr;50(3):390-396. doi: 10.1177/01926233221091747. Epub 2022 Apr 21. Toxicol Pathol. 2022. PMID: 35450478
-
Establishment of a patient-derived xenograft model and cell line of malignant transformation of mature cystic teratoma of the ovary.J Obstet Gynaecol Res. 2021 Feb;47(2):713-719. doi: 10.1111/jog.14596. Epub 2020 Dec 9. J Obstet Gynaecol Res. 2021. PMID: 33300248
-
The derivation of a Reference Dose (RfD) for perfluorooctane sulfonate (PFOS) based on immune suppression.Environ Res. 2019 Apr;171:452-469. doi: 10.1016/j.envres.2018.08.004. Epub 2018 Aug 8. Environ Res. 2019. PMID: 30739020 Review.
-
Perfluorooctane sulfonate: a review of human exposure, biomonitoring and the environmental forensics utility of its chirality and isomer distribution.Environ Int. 2015 Apr;77:148-59. doi: 10.1016/j.envint.2015.02.002. Epub 2015 Feb 27. Environ Int. 2015. PMID: 25728452 Review.
Cited by
-
The role of perfluorooctane sulfonic acid (PFOS) exposure in inflammation of intestinal tissues and intestinal carcinogenesis.Front Toxicol. 2023 Aug 15;5:1244457. doi: 10.3389/ftox.2023.1244457. eCollection 2023. Front Toxicol. 2023. PMID: 37662676 Free PMC article. Review.
-
SLCO1B3 promotes colorectal cancer tumorigenesis and metastasis through STAT3.Aging (Albany NY). 2021 Sep 15;13(18):22164-22175. doi: 10.18632/aging.203502. Epub 2021 Sep 15. Aging (Albany NY). 2021. PMID: 34526411 Free PMC article.
-
Establishment of a Patient-Derived Xenograft Model of Colorectal Cancer in CIEA NOG Mice and Exploring Smartfish Liquid Diet as a Source of Omega-3 Fatty Acids.Biomedicines. 2021 Mar 10;9(3):282. doi: 10.3390/biomedicines9030282. Biomedicines. 2021. PMID: 33802022 Free PMC article.
-
Perfluorooctane Sulfonate (PFOS) and Related Compounds Induce Nuclear Receptor 4A1 (NR4A1)-Dependent Carcinogenesis.Chem Res Toxicol. 2025 Apr 21;38(4):705-716. doi: 10.1021/acs.chemrestox.4c00528. Epub 2025 Mar 11. Chem Res Toxicol. 2025. PMID: 40066943 Free PMC article.
-
Per- and poly-fluoroalkyl substances exposure and risk of gastrointestinal cancers: a systematic review and meta-analysis.Eur J Cancer Prev. 2025 Sep 1;34(5):445-455. doi: 10.1097/CEJ.0000000000000935. Epub 2024 Dec 9. Eur J Cancer Prev. 2025. PMID: 39648934 Free PMC article.
References
-
- Ahlquist T, Lind GE, Costa VL, Meling GI, Vatn M, Hoff GS, Rognum TO, Skotheim RI, Thiis-Evensen E, Lothe RA. Gene methylation profiles of normal mucosa, and benign and malignant colorectal tumors identify early onset markers. Molecular Cancer. 2008;7:94. doi: 10.1186/1476-4598-7-94. - DOI - PMC - PubMed
-
- American Cancer Society . Colorectal cancer. American Cancer Society; Atlanta: 2017. pp. 1–63.
-
- Bertotti A, Migliardi G, Galimi F, Sassi F, Torti D, Isella C, Cora D, Di Nicolantonio F, Buscarino M, Petti C, Ribero D, Russolillo N, Muratore A, Massucco P, Pisacane A, Molinaro L, Valtorta E, Sartore-Bianchi A, Risio M, Capussotti L, Gambacorta M, Siena S, Medico E, Sapino A, Marsoni S, Comoglio PM, Bardelli A, Trusolino L. A molecularly annotated platform of patient-derived xenografts (“xenopatients”) identifies HER2 as an effective therapeutic target in cetuximab-resistant colorectal cancer. Cancer Discovery. 2011;1:508–523. doi: 10.1158/2159-8290.cd-11-0109. - DOI - PubMed
LinkOut - more resources
Full Text Sources

