Autophagy, EVs, and Infections: A Perfect Question for a Perfect Time
- PMID: 30406039
- PMCID: PMC6201680
- DOI: 10.3389/fcimb.2018.00362
Autophagy, EVs, and Infections: A Perfect Question for a Perfect Time
Abstract
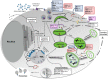
Autophagy, a highly conserved process, serves to maintain cellular homeostasis in response to an extensive variety of internal and external stimuli. The classic, or canonical, pathway of autophagy involves the coordinated degradation and recycling of intracellular components and pathogenic material. Proper regulation of autophagy is critical to maintain cellular health, as alterations in the autophagy pathway have been linked to the progression of a variety of physiological and pathological conditions in humans, namely in aging and in viral infection. In addition to its canonical role as a degradative pathway, a more unconventional and non-degradative role for autophagy has emerged as an area of increasing interest. This process, known as secretory autophagy, is gaining widespread attention as many viruses are believed to use this pathway as a means to release and spread viral particles. Moreover, secretory autophagy has been found to intersect with other intracellular pathways, such as the biogenesis and secretion of extracellular vesicles (EVs). Here, we provide a review of the current landscape surrounding both degradative autophagy and secretory autophagy in relation to both aging and viral infection. We discuss their key features, while describing their interplay with numerous different viruses (i.e. hepatitis B and C viruses, Epstein-Barr virus, SV40, herpesviruses, HIV, chikungunya virus, dengue virus, Zika virus, Ebola virus, HTLV, Rift Valley fever virus, poliovirus, and influenza A virus), and compare secretory autophagy to other pathways of extracellular vesicle release. Lastly, we highlight the need for, and emphasize the importance of, more thorough methods to study the underlying mechanisms of these pathways to better advance our understanding of disease progression.
Keywords: autophagy; exosome; extracellular vesicle; infectious disease; secretory autophagy; virus.
Figures
Similar articles
-
Extracellular Vesicle Release Promotes Viral Replication during Persistent HCV Infection.Cells. 2021 Apr 22;10(5):984. doi: 10.3390/cells10050984. Cells. 2021. PMID: 33922397 Free PMC article.
-
Extracellular Vesicles in Viral Infections of the Nervous System.Viruses. 2020 Jun 28;12(7):700. doi: 10.3390/v12070700. Viruses. 2020. PMID: 32605316 Free PMC article. Review.
-
Tetraspanin CD63 Bridges Autophagic and Endosomal Processes To Regulate Exosomal Secretion and Intracellular Signaling of Epstein-Barr Virus LMP1.J Virol. 2018 Feb 12;92(5):e01969-17. doi: 10.1128/JVI.01969-17. Print 2018 Mar 1. J Virol. 2018. PMID: 29212935 Free PMC article.
-
Autophagy and Mammalian Viruses: Roles in Immune Response, Viral Replication, and Beyond.Adv Virus Res. 2016;95:149-95. doi: 10.1016/bs.aivir.2016.02.002. Epub 2016 Mar 10. Adv Virus Res. 2016. PMID: 27112282 Review.
-
Escaping the Lion's Den: redirecting autophagy for unconventional release and spread of viruses.FEBS J. 2021 Jul;288(13):3913-3927. doi: 10.1111/febs.15590. Epub 2020 Oct 26. FEBS J. 2021. PMID: 33044763 Review.
Cited by
-
Extracellular Vesicles as New Players in Drug Delivery: A Focus on Red Blood Cells-Derived EVs.Pharmaceutics. 2023 Jan 21;15(2):365. doi: 10.3390/pharmaceutics15020365. Pharmaceutics. 2023. PMID: 36839687 Free PMC article. Review.
-
A Comprehensive Review of Autophagy and Its Various Roles in Infectious, Non-Infectious, and Lifestyle Diseases: Current Knowledge and Prospects for Disease Prevention, Novel Drug Design, and Therapy.Cells. 2019 Jul 3;8(7):674. doi: 10.3390/cells8070674. Cells. 2019. PMID: 31277291 Free PMC article. Review.
-
Differential traits between microvesicles and exosomes in enterovirus infection.MedComm (2020). 2023 Sep 24;4(5):e384. doi: 10.1002/mco2.384. eCollection 2023 Oct. MedComm (2020). 2023. PMID: 37752943 Free PMC article.
-
Autophagy and Aging: Roles in Skeletal Muscle, Eye, Brain and Hepatic Tissue.Front Cell Dev Biol. 2021 Oct 28;9:752962. doi: 10.3389/fcell.2021.752962. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34778264 Free PMC article. Review.
-
Extracellular vesicles from HTLV-1 infected cells modulate target cells and viral spread.Retrovirology. 2021 Feb 23;18(1):6. doi: 10.1186/s12977-021-00550-8. Retrovirology. 2021. PMID: 33622348 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

