Multicomponent Reactions Accelerated by Aqueous Micelles
- PMID: 30406083
- PMCID: PMC6204348
- DOI: 10.3389/fchem.2018.00502
Multicomponent Reactions Accelerated by Aqueous Micelles
Abstract
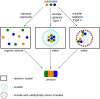
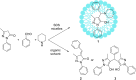
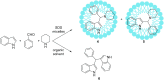
Multicomponent reactions are powerful synthetic tools for the efficient creation of complex organic molecules in an one-pot one-step fashion. Moreover, the amount of solvents and energy needed for separation and purification of intermediates is significantly reduced what is beneficial from the green chemistry issues point of view. This review highlights the development of multicomponent reactions conducted using aqueous micelles systems during the last two decades.
Keywords: micelles; multicomponent reactions; on water reaction; surfactants; water.
Figures




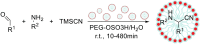
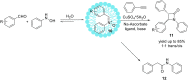
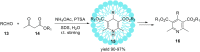





References
-
- Akiyama T., Matsuda K., Fuchibe K. (2005). HCl-Catalyzed Stereoselective Mannich Reaction in H2O–SDS System. Synlett 2, 322–324. 10.1055/s-2004-836062 - DOI
-
- Armenise N., Malferrari D., Ricciardulli S., Galletti P., Tangliavini E. (2016). Multicomponent cascade synthesis of biaryl-based chalcones in pure water and in an aqueous micellar environment. Eur. J. Org. Chem. 3177–3185. 10.1002/ejoc.201600095 - DOI
Publication types
LinkOut - more resources
Full Text Sources

