Multicellular Interactions in 3D Engineered Myocardial Tissue
- PMID: 30406114
- PMCID: PMC6205951
- DOI: 10.3389/fcvm.2018.00147
Multicellular Interactions in 3D Engineered Myocardial Tissue
Abstract
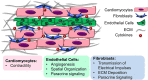
Cardiovascular disease is a leading cause of death in the US and many countries worldwide. Current cell-based clinical trials to restore cardiomyocyte (CM) health by local delivery of cells have shown only moderate benefit in improving cardiac pumping capacity. CMs have highly organized physiological structure and interact dynamically with non-CM populations, including endothelial cells and fibroblasts. Within engineered myocardial tissue, non-CM populations play an important role in CM survival and function, in part by secreting paracrine factors and cell-cell interactions. In this review, we summarize the progress of engineering myocardial tissue with pre-formed physiological multicellular organization, and present the challenges toward clinical translation.
Keywords: cardiomyocyte; cardiovascular tissue engineering; co-culture; endothelial cell; engineered myocardium; fibroblast; stem cell.
Figures
Similar articles
-
Age-dependent functional crosstalk between cardiac fibroblasts and cardiomyocytes in a 3D engineered cardiac tissue.Acta Biomater. 2017 Jun;55:120-130. doi: 10.1016/j.actbio.2017.04.027. Epub 2017 Apr 25. Acta Biomater. 2017. PMID: 28455218 Free PMC article.
-
Engineered myocardium model to study the roles of HIF-1α and HIF1A-AS1 in paracrine-only signaling under pathological level oxidative stress.Acta Biomater. 2017 Aug;58:323-336. doi: 10.1016/j.actbio.2017.06.023. Epub 2017 Jun 16. Acta Biomater. 2017. PMID: 28629892 Free PMC article.
-
Single-Cell Determination of Cardiac Microtissue Structure and Function Using Light Sheet Microscopy.Tissue Eng Part C Methods. 2020 Apr;26(4):207-215. doi: 10.1089/ten.TEC.2020.0020. Epub 2020 Apr 3. Tissue Eng Part C Methods. 2020. PMID: 32111148
-
Heart regeneration with engineered myocardial tissue.Annu Rev Biomed Eng. 2014 Jul 11;16:1-28. doi: 10.1146/annurev-bioeng-071812-152344. Epub 2014 Apr 24. Annu Rev Biomed Eng. 2014. PMID: 24819474 Free PMC article. Review.
-
Naturally Engineered Maturation of Cardiomyocytes.Front Cell Dev Biol. 2017 May 5;5:50. doi: 10.3389/fcell.2017.00050. eCollection 2017. Front Cell Dev Biol. 2017. PMID: 28529939 Free PMC article. Review.
Cited by
-
Functional human cell-based vascularised cardiac tissue model for biomedical research and testing.Sci Rep. 2022 Aug 5;12(1):13459. doi: 10.1038/s41598-022-17498-0. Sci Rep. 2022. PMID: 35931748 Free PMC article.
-
Advances in 3D Organoid Models for Stem Cell-Based Cardiac Regeneration.Int J Mol Sci. 2023 Mar 8;24(6):5188. doi: 10.3390/ijms24065188. Int J Mol Sci. 2023. PMID: 36982261 Free PMC article. Review.
-
Cell spheroid fusion: beyond liquid drops model.Sci Rep. 2020 Jul 28;10(1):12614. doi: 10.1038/s41598-020-69540-8. Sci Rep. 2020. PMID: 32724115 Free PMC article.
-
Human Cell Modeling for Cardiovascular Diseases.Int J Mol Sci. 2020 Sep 2;21(17):6388. doi: 10.3390/ijms21176388. Int J Mol Sci. 2020. PMID: 32887493 Free PMC article. Review.
-
Human cardiac fibroblasts expressing VCAM1 improve heart function in postinfarct heart failure rat models by stimulating lymphangiogenesis.PLoS One. 2020 Sep 16;15(9):e0237810. doi: 10.1371/journal.pone.0237810. eCollection 2020. PLoS One. 2020. PMID: 32936824 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources