30-day mortality after the start of systemic anticancer therapy for lung cancer: is it really a useful performance indicator?
- PMID: 30406123
- PMCID: PMC6215912
- DOI: 10.1183/23120541.00030-2018
30-day mortality after the start of systemic anticancer therapy for lung cancer: is it really a useful performance indicator?
Abstract
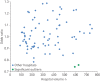
Systemic treatment is the standard treatment for unresectable stage III and IV lung cancer. Nevertheless, a 5-10% death rate has been described within 30 days after the last systemic treatment, suggesting that these patient did not benefit. We analysed the 30-day mortality after start of systemic therapy. Data were retrieved from the Netherlands National Cancer Registry. From 2010 to 2015, 26 277 patients were included. 56% were men. The median age was 65 years and 31% of patients were aged ≥70 years. 27% involved small cell lung cancer and 73% nonsmall cell lung cancer. Overall mortality within 30 days after the start of systemic treatment was 6.2%. Multivariable analysis established the prognostic influence of age, histology, number of metastatic sites and type of systemic treatment. Chemotherapy was administered in 77 hospitals, treating each 15-161 lung cancer patients with systemic therapy annually. None of the hospitals had a significantly higher 30-day mortality according to hierarchical multivariable analysis, controlling for case-mix. In the Netherlands, the 30-day mortality rate after start of systemic therapy for lung cancer patients was comparable with earlier reports. Hospital volume did not influence the 30-day mortality rate. 30-day mortality rate is not a meaningful indicator to monitor quality of care.
Conflict of interest statement
Conflict of interest: J.A. Burgers reports having served on advisory boards for Boehringer Ingelheim, AstraZeneca and Roche outside the submitted work. Conflict of interest: R.A. Damhuis has nothing to disclose.
Figures
References
-
- Mok TS, Wu YL, Thongprasert S, et al. . Gefitinib or carboplatin–paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009; 361: 947–587. - PubMed
-
- Shepherd FA, Dancey J, Ramlau R, et al. . Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol 2000; 18: 2095–2103. - PubMed
LinkOut - more resources
Full Text Sources