Diagnostic performance of Elecsys immunoassays for cerebrospinal fluid Alzheimer's disease biomarkers in a nonacademic, multicenter memory clinic cohort: The ABIDE project
- PMID: 30406175
- PMCID: PMC6215060
- DOI: 10.1016/j.dadm.2018.08.006
Diagnostic performance of Elecsys immunoassays for cerebrospinal fluid Alzheimer's disease biomarkers in a nonacademic, multicenter memory clinic cohort: The ABIDE project
Abstract
Introduction: We compared the automated Elecsys and manual Innotest immunoassays for cerebrospinal fluid (CSF) Alzheimer's disease biomarkers in a multicenter diagnostic setting.
Methods: We collected CSF samples from 137 participants in eight local memory clinics. Amyloid β(1-42) (Aβ42), total tau (t-tau), and phosphorylated tau (p-tau) were centrally analyzed with Innotest and Elecsys assays. Concordances between methods were assessed.
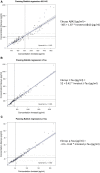
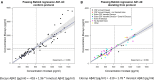
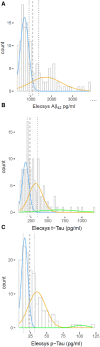
Results: Biomarker results strongly correlated between assays with Spearman's ρ 0.94 for Aβ42, 0.98 for t-tau, and 0.98 for p-tau. Using Gaussian mixture modeling, cohort-specific cut-points were estimated at 1092 pg/mL for Aβ42, 235 pg/mL for t-tau, and 24 pg/mL for p-tau. We found an excellent concordance of biomarker abnormality between assays of 97% for Aβ42 and 96% for both t-tau and p-tau.
Discussion: The high concordances between Elecsys and Innotest in this nonacademic, multicenter cohort support the use of Elecsys for CSF Alzheimer's disease diagnostics and allow conversion of results between methods.
Keywords: Alzheimer's disease; Biomarkers; Cerebrospinal fluid; Clinical setting; Conversion formula; Elecsys; Gaussian mixture modeling; Immunoassay; Innotest; Method comparison; Multicenter study; Nonacademic cohort.
Figures

Similar articles
-
Alzheimer's cerebrospinal biomarkers from Lumipulse fully automated immunoassay: concordance with amyloid-beta PET and manual immunoassay in Koreans : CSF AD biomarkers measured by Lumipulse in Koreans.Alzheimers Res Ther. 2021 Jan 12;13(1):22. doi: 10.1186/s13195-020-00767-3. Alzheimers Res Ther. 2021. PMID: 33436035 Free PMC article.
-
Assessment of the Concordance and Diagnostic Accuracy Between Elecsys and Lumipulse Fully Automated Platforms and Innotest.Front Aging Neurosci. 2021 Mar 4;13:604119. doi: 10.3389/fnagi.2021.604119. eCollection 2021. Front Aging Neurosci. 2021. PMID: 33746733 Free PMC article.
-
Concordance Between Different Amyloid Immunoassays and Visual Amyloid Positron Emission Tomographic Assessment.JAMA Neurol. 2017 Dec 1;74(12):1492-1501. doi: 10.1001/jamaneurol.2017.2814. JAMA Neurol. 2017. PMID: 29114726 Free PMC article.
-
Fluid biomarker-based molecular phenotyping of Alzheimer's disease patients in research and clinical settings.Prog Mol Biol Transl Sci. 2019;168:3-23. doi: 10.1016/bs.pmbts.2019.07.006. Epub 2019 Jul 24. Prog Mol Biol Transl Sci. 2019. PMID: 31699324 Review.
-
Biomarkers for Alzheimer's disease: current status and prospects for the future.J Intern Med. 2018 Dec;284(6):643-663. doi: 10.1111/joim.12816. Epub 2018 Aug 19. J Intern Med. 2018. PMID: 30051512 Review.
Cited by
-
Time course of phosphorylated-tau181 in blood across the Alzheimer's disease spectrum.Brain. 2021 Feb 12;144(1):325-339. doi: 10.1093/brain/awaa399. Brain. 2021. PMID: 33257949 Free PMC article.
-
Synaptic biomarkers in the cerebrospinal fluid associate differentially with classical neuronal biomarkers in patients with Alzheimer's disease and frontotemporal dementia.Alzheimers Res Ther. 2023 Mar 24;15(1):62. doi: 10.1186/s13195-023-01212-x. Alzheimers Res Ther. 2023. PMID: 36964594 Free PMC article.
-
Differential diagnostic performance of a panel of plasma biomarkers for different types of dementia.Alzheimers Dement (Amst). 2022 May 15;14(1):e12285. doi: 10.1002/dad2.12285. eCollection 2022. Alzheimers Dement (Amst). 2022. PMID: 35603139 Free PMC article.
-
An accurate fully automated panel of plasma biomarkers for Alzheimer's disease.Alzheimers Dement. 2023 Apr;19(4):1204-1215. doi: 10.1002/alz.12751. Epub 2022 Aug 11. Alzheimers Dement. 2023. PMID: 35950735 Free PMC article.
-
Comparing CSF amyloid-beta biomarker ratios for two automated immunoassays, Elecsys and Lumipulse, with amyloid PET status.Alzheimers Dement (Amst). 2021 May 1;13(1):e12182. doi: 10.1002/dad2.12182. eCollection 2021. Alzheimers Dement (Amst). 2021. PMID: 33969174 Free PMC article.
References
-
- Galasko D., Chang L., Motter R., Clark C.M., Kaye J., Knopman D. High cerebrospinal fluid tau and low amyloid b42 levels in the clinical diagnosis of Alzheimer disease and relation to apolipoprotein E genotype. Arch Neurol. 1998;55:937–945. - PubMed
-
- Scheltens P., Blennow K., Breteler M.M.B., de Strooper B., Frisoni G.B., Salloway S. Alzheimer's disease. Lancet. 2016;388:505–517. - PubMed
-
- Dubois B., Feldman H.H., Jacova C., Hampel H., Molinuevo J.L., Blennow K. Advancing research diagnostic criteria for Alzheimer's disease: the IWG-2 criteria. Lancet Neurol. 2014;13:614–629. - PubMed
-
- Mattsson N., Zetterberg H., Hansson O., Andreasen N., Parnetti L., Jonsson M. CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment. JAMA. 2009;302:385–393. - PubMed
-
- Frisoni G.B., Boccardi M., Barkhof F., Blennow K., Cappa S., Chiotis K. Strategic roadmap for an early diagnosis of Alzheimer's disease based on biomarkers. Lancet Neurol. 2017;16:661–676. - PubMed
LinkOut - more resources
Full Text Sources
