Neuropharmacology of Synthetic Cathinones
- PMID: 30406443
- PMCID: PMC7257813
- DOI: 10.1007/164_2018_178
Neuropharmacology of Synthetic Cathinones
Abstract
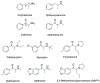
Synthetic cathinones are derivatives of the naturally occurring compound cathinone, the main psychoactive ingredient in the khat plant Catha edulis. Cathinone is the β-keto analog of amphetamine, and all synthetic cathinones display a β-keto moiety in their structure. Several synthetic cathinones are widely prescribed medications (e.g., bupropion, Wellbutrin®), while others are problematic drugs of abuse (e.g., 4-methylmethcathinone, mephedrone). Similar to amphetamines, synthetic cathinones are psychomotor stimulants that exert their effects by impairing the normal function of plasma membrane transporters for dopamine (DAT), norepinephrine (NET), and 5-HT (SERT). Ring-substituted cathinones like mephedrone are transporter substrates that evoke neurotransmitter release by reversing the normal direction of transporter flux (i.e., releasers), whereas pyrrolidine-containing cathinones like 3,4-methylenedioxypyrovalerone (MDPV) are potent transporter inhibitors that block neurotransmitter uptake (i.e., blockers). Regardless of molecular mechanism, all synthetic cathinones increase extracellular monoamine concentrations in the brain, thereby enhancing cell-to-cell monoamine signaling. Here, we briefly review the mechanisms of action, structure-activity relationships, and in vivo pharmacology of synthetic cathinones. Overall, the findings show that certain synthetic cathinones are powerful drugs of abuse that could pose significant risk to users.
Keywords: Cathinone; Dopamine; Monoamine; Serotonin; Stimulant; Transporter.
Figures


References
-
- Aarde SM, Creehan KM, Vandewater SA, Dickerson TJ, Taffe MA (2015) In vivo potency and efficacy of the novel cathinone α-pyrrolidinopentiophenone and 3,4-methylenedioxypyrovalerone: self-administration and locomotor stimulation in male rats. Psychopharmacology (Berl) 232 (16):3045–3055 - PMC - PubMed
-
- Al-Hebshi NN, Skaugh N (2005) Khat (Catha edulis)- an updated review. Addict Biol 10 (4):299–307 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

