The High Chemofidelity of Metal-Catalyzed Hydrogen Atom Transfer
- PMID: 30406655
- PMCID: PMC6248883
- DOI: 10.1021/acs.accounts.8b00337
The High Chemofidelity of Metal-Catalyzed Hydrogen Atom Transfer
Abstract
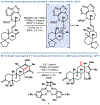
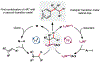
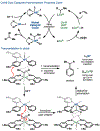
The implementation of any chemical reaction in a structurally complex setting ( King , S. M. J. Org. Chem. 2014 , 79 , 8937 ) confronts structurally defined barriers: steric environment, functional group reactivity, product instability, and through-bond electronics. However, there are also practical barriers. Late-stage reactions conducted on small quantities of material are run inevitably at lower than optimal concentrations. Access to late-stage material limits extensive optimization. Impurities from past reactions can interfere, especially with catalytic reactions. Therefore, chemical reactions on which one can rely at the front lines of a complex synthesis campaign emerge from the crucible of total synthesis as robust, dependable, and widely applied. Trost conceptualized "chemoselectivity" as a reagent's selective reaction of one functional group or reactive site in preference to others ( Trost , B. M. Science 1983 , 219 , 245 ). Chemoselectivity and functional group tolerance can be evaluated quickly using robustness screens ( Collins , K. D. Nat. Chem. 2013 , 5 , 597 ). A reaction may also be characterized by its "chemofidelity", that is, its reliable reaction with a functional group in any molecular context. For example, ketone reduction by an electride (dissolving metal conditions) exhibits high chemofidelity but low chemoselectivity: it usually works, but many other functional groups are reduced at similar rates. Conversely, alkene coordination chemistry effected by π Lewis acids can exhibit high chemoselectivity ( Trost , B. M. Science 1983 , 219 , 245 ) but low chemofidelity: it can be highly selective for alkenes but sensitive to the substitution pattern ( Larionov , E. Chem. Commun. 2014 , 50 , 9816 ). In contrast, alkenes undergo reliable, robust, and diverse hydrogen atom transfer reactions from metal hydrides to generate carbon-centered radicals. Although there are many potential applications of this chemistry, its functional group tolerance, high rates, and ease of execution have led to its rapid deployment in complex synthesis campaigns. Its success derives from high chemofidelity, that is, its dependable reactivity in many molecular environments and with many alkene substitution patterns. Metal hydride H atom transfer (MHAT) reactions convert diverse, simple building blocks to more stereochemically and functionally dense products ( Crossley , S. W. M. Chem. Rev. 2016 , 116 , 8912 ). When hydrogen is returned to the metal, MHAT can be considered the radical equivalent of Brønsted acid catalysis-itself a broad reactivity paradigm. This Account summarizes our group's contributions to method development, reagent discovery, and mechanistic interrogation. Our earliest contribution to this area-a stepwise hydrogenation with high chemoselectivity and high chemofidelity-has found application to many problems. More recently, we reported the first examples of dual-catalytic cross-couplings that rely on the merger of MHAT cycles and nickel catalysis. With time, we anticipate that MHAT will become a staple of chemical synthesis.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



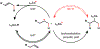




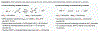

References
-
- Choi J; Tang L; Norton JR Kinetics of Hydrogen Atom Transfer from (η5-C5H5)Cr(CO)3H to Various Olefins: Influence of Olefin Structure. J. Am. Chem. Soc 2007, 129, 234–240. - PubMed
-
- King SM; Ma X; Herzon SB A Method for the Selective Hydrogenation of Alkenyl Halides to Alkyl Halides. J. Am. Chem. Soc 2014, 136, 6884–6887. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

