Long-term safety and immunogenicity of the M72/AS01E candidate tuberculosis vaccine in HIV-positive and -negative Indian adults: Results from a phase II randomized controlled trial
- PMID: 30407329
- PMCID: PMC6250513
- DOI: 10.1097/MD.0000000000013120
Long-term safety and immunogenicity of the M72/AS01E candidate tuberculosis vaccine in HIV-positive and -negative Indian adults: Results from a phase II randomized controlled trial
Erratum in
-
Long-term safety and immunogenicity of the M72/AS01E candidate tuberculosis vaccine in HIV-positive and -negative Indian adults: Results from a phase II randomized controlled trial: Erratum.Medicine (Baltimore). 2018 Dec;97(52):e13948. doi: 10.1097/MD.0000000000013948. Medicine (Baltimore). 2018. PMID: 30593216 Free PMC article. No abstract available.
Abstract
Objectives: To assess the long-term safety and immunogenicity of the M72/ Adjuvant System (AS01E) candidate tuberculosis (TB) vaccine up to 3 years post-dose 2 (Y3) in human immunodeficiency virus (HIV)-positive (HIV+) and HIV-negative (HIV-) Indian adults.
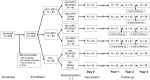
Methods: This phase II, double-blind, randomised, controlled clinical trial (NCT01262976) was conducted at YRG CARE Medical Centre, in Chennai, India, between January 2011 and June 2015.Three cohorts (HIV+ participants stable on antiretroviral therapy [ART; HIV+ART+], HIV+ ART-naïve [HIV+ART-], and HIV- participants) were randomised (1:1) to receive 2 doses of M72/AS01E (M72/AS01E groups) or saline (control groups) 1 month apart and were followed up toY3. Latent TB infection was assessed at screening using an interferon-gamma (IFN-γ) release assay (IGRA). Safety and immunogenicity results up to Y1 post-vaccination were reported elsewhere. Here, we report serious adverse events (SAEs), humoral and cell-mediated immune (CMI) responses to M72 recorded at Y2 and Y3.
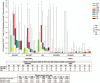
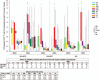
Results: Of 240 enrolled and vaccinated participants, 214 completed the long-term follow-up part of the study.In addition to SAEs previously described, between Y1 and Y2 1 M72/AS01E recipient in the HIV+ART+ cohort reported 2 SAEs (sinus cavernous thrombosis and gastroenteritis) that were not considered as causally related to the study vaccine.Vaccination elicited persistent humoral immune responses against M72. At Y3, seropositivity rates were 97.1%, 66.7%, and 97.3% and geometric mean concentrations (GMCs) were 22.0 ELISA units (EU)/mL, 4.9 EU/mL, and 24.3 EU/mL in the HIV+ART+, HIV+ART-, and HIV- cohorts, respectively. Humoral immune response was lowest in the HIV+ART- cohort.In M72/AS01E recipients, no notable decrease in the frequency of M72-specific CD4 T-cells expressing ≥2 immune markers among interleukin-2 (IL-2), IFN-γ, tumour necrosis factor alpha (TNF-α) and CD40 ligand (CD40L) was observed at Y3 post-vaccination. Median values (interquartile range) of 0.35% (0.13-0.49), 0.05% (0.01-0.10), and 0.15% (0.09-0.22) were recorded in the HIV+ART+, HIV+ART- and HIV- cohorts, respectively. CD4 T-cell response was lowest in the HIV+ART- cohort.No CD8 T-cell response was observed.
Conclusion: The cellular and humoral immune responses induced by M72/AS01E in HIV+ and HIV- adults persisted up to Y3 post-vaccination. No safety concerns were raised regarding administration of M72/AS01E to HIV+ adults.
Clinical trial registration: NCT01262976 (www.clinicaltrials.gov).
Conflict of interest statement
The authors report no other conflicts of interest
Figures






References
-
- Tiberi S, Carvalho AC, Sulis G, et al. The cursed duet today: tuberculosis and HIV-coinfection. Presse Med 2017;46:e23–39. - PubMed
-
- WHO. Global tuberculosis report 2017. Geneva: World Health Organization; 2017.
-
- von Reyn CF, Bakari M, Arbeit RD, et al. New vaccines for the prevention of tuberculosis in human immunodeficiency virus infection. Int J Tuberc Lung Dis 2012;16:718–23. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

