Challenges and Opportunities of Centrifugal Microfluidics for Extreme Point-of-Care Testing
- PMID: 30407405
- PMCID: PMC6190358
- DOI: 10.3390/mi7020032
Challenges and Opportunities of Centrifugal Microfluidics for Extreme Point-of-Care Testing
Abstract
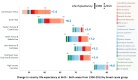
The advantages offered by centrifugal microfluidic systems have encouraged its rapid adaptation in the fields of in vitro diagnostics, clinical chemistry, immunoassays, and nucleic acid tests. Centrifugal microfluidic devices are currently used in both clinical and point-of-care settings. Recent studies have shown that this new diagnostic platform could be potentially used in extreme point-of-care settings like remote villages in the Indian subcontinent and in Africa. Several technological inventions have decentralized diagnostics in developing countries; however, very few microfluidic technologies have been successful in meeting the demand. By identifying the finest difference between the point-of-care testing and extreme point-of-care infrastructure, this review captures the evolving diagnostic needs of developing countries paired with infrastructural challenges with technological hurdles to healthcare delivery in extreme point-of-care settings. In particular, the requirements for making centrifugal diagnostic devices viable in developing countries are discussed based on a detailed analysis of the demands in different clinical settings including the distinctive needs of extreme point-of-care settings.
Keywords: centrifugal microfluidics; clinical chemistry; developing countries; diagnostics; immunoassays; nucleic acid tests; point-of-care.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Ducrée J., Haeberle S., Brenner T., Glatzel T., Zengerle R. Patterning of flow and mixing in rotating radial microchannels. Microfluid. Nanofluid. 2006;2:97–105. doi: 10.1007/s10404-005-0049-4. - DOI
-
- Madou M.J., Kellogg G.J. Labcd: A centrifuge-based microfluidic platform for diagnostics; SPIE Proceedings of the Systems and Technologies for Clinical Diagnostics and Drug Discovery; San Jose, CA, USA. 27 January 1998; pp. 80–93.
Publication types
LinkOut - more resources
Full Text Sources

