Allergy Testing and Drug Screening on an ITO-Coated Lab-on-a-Disc
- PMID: 30407411
- PMCID: PMC6189934
- DOI: 10.3390/mi7030038
Allergy Testing and Drug Screening on an ITO-Coated Lab-on-a-Disc
Abstract
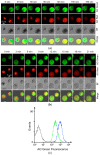
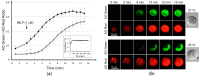
A lab-on-a-disc (LOAD) is a centrifugal microfluidic set-up based on centrifugal force without using micro-pumps to drive reagents and cells to various chambers through channels and valves for reactions. A LOAD coated with conductive transparent indium tin oxide (ITO) for thermal control was developed to screen allergy-blocking agents. When the acridine orange (AO)-loaded KU-812 human basophilic cells were activated in the LOAD by stimuli, AO trapped in the cytoplasmic granules was released externally as an allergic mediator mimetic to report degranulation. This response was monitored by fluorescence when the released AO in supernatant had been transferred, with a higher spinning speed, from the reaction chamber to detection chamber in the LOAD where AO reacted with exogenous DNA. We report here the principles of the system and an improved LOAD set-up with the ITO-coated glass resistive microheater to run assays at 37 °C. By using this platform, we demonstrate here for the first time that triptolide, an active ingredient from the Chinese medicine herb Tripterygium wilfordii Hook f., was able to suppress the fMLP-mediated degranulation in basophils. This serves as an example how LOADs can be used to screen agents to alleviate symptoms of allergy.
Keywords: N-formyl-methionine-leucine-phenylalanine (fMLP); Traditional Chinese Medicine (TCM); World Allergy Organization (WAO); acridine orange (AO); basophil activation test (BAT); bisphenol A (BPA); indium tin oxide (ITO); lab-on-a-disc (LOAD); phorbol 12-myristate 13-acetate (PMA); protein kinase C (PKC).
Conflict of interest statement
All authors declare no conflict of interest.
Figures



References
-
- WAO White Book 2013. [(accessed on 7 December 2015)]. Available online: http://www.worldallergy.org/definingthespecialty/white_book.php.
-
- Lambert C., Sarrat A., Bienvenu F., Brabant S., Nicaise-Roland P., Alyanakian M.A., Apoil P.A., Capron C., Couderc R., Evrard B., et al. The importance of EN ISO 15189 accreditation of allergen-specific IgE determination for reliable in vitro allergy diagnosis. Allergy. 2015;70:180–186. doi: 10.1111/all.12546. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

