Optofluidic Device Based Microflow Cytometers for Particle/Cell Detection: A Review
- PMID: 30407441
- PMCID: PMC6189758
- DOI: 10.3390/mi7040070
Optofluidic Device Based Microflow Cytometers for Particle/Cell Detection: A Review
Abstract
Optofluidic devices combining micro-optical and microfluidic components bring a host of new advantages to conventional microfluidic devices. Aspects, such as optical beam shaping, can be integrated on-chip and provide high-sensitivity and built-in optical alignment. Optofluidic microflow cytometers have been demonstrated in applications, such as point-of-care diagnostics, cellular immunophenotyping, rare cell analysis, genomics and analytical chemistry. Flow control, light guiding and collecting, data collection and data analysis are the four main techniques attributed to the performance of the optofluidic microflow cytometer. Each of the four areas is discussed in detail to show the basic principles and recent developments. 3D microfabrication techniques are discussed in their use to make these novel microfluidic devices, and the integration of the whole system takes advantage of the miniaturization of each sub-system. The combination of these different techniques is a spur to the development of microflow cytometers, and results show the performance of many types of microflow cytometers developed recently.
Keywords: microfabrication; microflow cytometer; microfluidics; optofluidic device.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

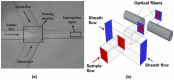




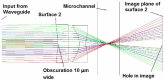


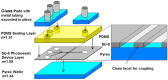
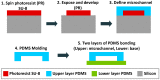
References
-
- Horowitz V.R., Awschalow D.D., Pennathur S. Optofluidics: Field or technique? Lab Chip. 2008;8:1856–1863. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

