Cannabis for the treatment of Crohn's disease
- PMID: 30407616
- PMCID: PMC6517156
- DOI: 10.1002/14651858.CD012853.pub2
Cannabis for the treatment of Crohn's disease
Abstract
Background: Crohn's disease (CD) is a chronic immune-mediated condition of transmural inflammation in the gastrointestinal tract, associated with significant morbidity and decreased quality of life. The endocannabinoid system provides a potential therapeutic target for cannabis and cannabinoids and animal models have shown benefit in decreasing inflammation. However, there is also evidence to suggest transient adverse events such as weakness, dizziness and diarrhea, and an increased risk of surgery in people with CD who use cannabis.
Objectives: The objectives were to assess the efficacy and safety of cannabis and cannabinoids for induction and maintenance of remission in people with CD.
Search methods: We searched MEDLINE, Embase, AMED, PsychINFO, the Cochrane IBD Group Specialized Register, CENTRAL, ClinicalTrials.Gov, and the European Clinical Trials Register up to 17 October 2018. We searched conference abstracts, references and we also contacted researchers in this field for upcoming publications.
Selection criteria: Randomized controlled trials comparing any form of cannabis or its cannabinoid derivatives (natural or synthetic) to placebo or an active therapy for adults with Crohn's disease were included.
Data collection and analysis: Two authors independently screened search results, extracted data and assessed bias using the Cochrane risk of bias tool. The primary outcomes were clinical remission and relapse. Remission is commonly defined as a Crohn's disease activity index (CDAI) of < 150. Relapse is defined as a CDAI > 150. Secondary outcomes included clinical response, endoscopic remission, endoscopic improvement, histological improvement, quality of life, C-reactive protein (CRP) and fecal calprotectin measurements, adverse events (AEs), serious AEs, withdrawal due to AEs, and cannabis dependence and withdrawal effects. We calculated the risk ratio (RR) and corresponding 95% confidence interval (95% CI) for dichotomous outcomes. For continuous outcomes, we calculated the mean difference (MD) and 95% CI. Data were combined for analysis when the interventions, patient groups and outcomes were sufficiently similar (determined by consensus). Data were analyzed on an intention-to-treat basis and the overall certainty of the evidence supporting the outcomes was evaluated using the GRADE criteria.
Main results: Three studies (93 participants) that assessed cannabis in people with active CD met the inclusion criteria. One ongoing study was also identified. Participants in two of the studies were adults with active Crohn's disease who had failed at least one medical treatment. The inclusion criteria for the third study were unclear. No studies that assessed cannabis therapy in quiescent CD were identified. The studies were not pooled due to differences in the interventional drug.One small study (N = 21) compared eight weeks of treatment with cannabis cigarettes containing 115 mg of D9-tetrahydrocannabinol (THC) to placebo cigarettes containing cannabis with the THC removed in participants with active CD. This study was rated as high risk of bias for blinding and other bias (cannabis participants were older than placebo). The effects of cannabis on clinical remission were unclear. Forty-five per cent (5/11) of the cannabis group achieved clinical remission compared with 10% (1/10) of the placebo group (RR 4.55, 95% CI 0.63 to 32.56; very low certainty evidence). A difference was observed in clinical response (decrease in CDAI score of >100 points) rates. Ninety-one per cent (10/11) of the cannabis group achieved a clinical response compared to 40% (4/10) of the placebo group (RR 2.27, 95% CI 1.04 to 4.97; very low certainty evidence). More AEs were observed in the cannabis cigarette group compared to placebo (RR 4.09, 95% CI 1.15 to 14.57; very low certainty evidence). These AEs were considered to be mild in nature and included sleepiness, nausea, difficulty with concentration, memory loss, confusion and dizziness. This study did not report on serious AEs or withdrawal due to AEs.One small study (N = 22) compared cannabis oil (5% cannabidiol) to placebo oil in people with active CD. This study was rated as high risk of bias for other bias (cannabis participants were more likely than placebo participants to be smokers). There was no difference in clinical remission rates. Forty per cent (4/10) of cannabis oil participants achieved remission at 8 weeks compared to 33% (3/9) of the placebo participants (RR 1.20, 95% CI 0.36 to 3.97; very low certainty evidence). There was no difference in the proportion of participants who had a serious adverse event. Ten per cent (1/10) of participants in the cannabis oil group had a serious adverse event compared to 11% (1/9) of placebo participants (RR 0.90, 95% CI 0.07 to 12.38, very low certainty evidence). Both serious AEs were worsening Crohn's disease that required rescue intervention. This study did not report on clinical response, CRP, quality of life or withdrawal due to AEs.One small study (N= 50) compared cannabis oil (15% cannabidiol and 4% THC) to placebo in participants with active CD. This study was rated as low risk of bias. Differences in CDAI and quality of life scores measured by the SF-36 instrument were observed. The mean quality of life score after 8 weeks of treatment was 96.3 in the cannabis oil group compared to 79.9 in the placebo group (MD 16.40, 95% CI 5.72 to 27.08, low certainty evidence). After 8 weeks of treatment, the mean CDAI score was118.6 in the cannabis oil group compared to 212.6 in the placebo group (MD -94.00, 95%CI -148.86 to -39.14, low certainty evidence). This study did not report on clinical remission, clinical response, CRP or AEs.
Authors' conclusions: The effects of cannabis and cannabis oil on Crohn's disease are uncertain. Thus no firm conclusions regarding the efficacy and safety of cannabis and cannabis oil in adults with active Crohn's disease can be drawn. The effects of cannabis or cannabis oil in quiescent Crohn's disease have not been investigated. Further studies with larger numbers of participants are required to assess the potential benefits and harms of cannabis in Crohn's disease. Future studies should assess the effects of cannabis in people with active and quiescent Crohn's disease. Different doses of cannabis and delivery modalities should be investigated.
Conflict of interest statement
Tahir S Kafil: None known
Tran M Nguyen: None known
John K MacDonald: None known
Nilesh Chande has received funds from AbbVie, Ferring, and Takdeda for consulting; and payment for lectures from Abbvie and Actavis. All of these financial activities are outside the submitted work.
Figures
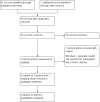









Update of
References
References to studies included in this review
Naftali 2013a {published and unpublished data}
-
- Naftali T, Bar‐Lev L, Gabay G, Chowers Y, Dotan I, Bronshtein M, et al. Tetrahydrocannabinol (THC) rich medical cannabis induces clinical and biochemical improvement with a steroid sparing effect in active Crohn's disease. Gastroenterology 2012;1:S780.
-
- Naftali T, Bar‐Lev Schleider L, Dotan I, Lansky EP, Sklerovsky Benjaminov F, Konikoff FM. Cannabis induces a clinical response in patients with Crohn’s disease: a prospective placebo‐controlled study. Clinical Gastroenterology and Hepatology 2013;11(10):1276‐80. - PubMed
Naftali 2017a {published and unpublished data}
-
- Naftali T, Mechoulam R, Gabay G, Stein A, Bronshtein M, Mari A, et al. Cannabidiol treatment does not effect active Crohn's disease. Gastroenterology 2013;1:S180.
-
- Naftali T, Mechulam R, Marii A, Gabay G, Stein A, Bronshtain M, et al. Low‐dose cannabidiol is safe but not effective in the treatment for Crohn’s Disease, a randomized controlled trial. Digestive Diseases and Sciences 2017;62:1615–20. - PubMed
Naftali 2017b {published and unpublished data}
-
- Naftali T, Schlieder LBL, Konikoff FM. The effect of cannabis on Crohn's Disease patients. International Association of Cannabis Medicine (IACM). 2017.
References to studies excluded from this review
Naftali 2013b {published and unpublished data}
-
- Naftali T, Barlev L, Gabay G, Chowers Y, Dotan I, Stein A, et al. Tetrahydrocannabinol (THC) induces clinical and biochemical improvement with a steroid sparing effect in active inflammatory bowel disease. Journal of Crohn's and Colitis 2013;7(Supplement1):S153.
References to ongoing studies
NCT03467620 {published data only}
-
- NCT03467620. Cannabidiol Usage as an Adjunct Therapy for Crohn's Disease [Oral Cannabidiol Capsule Usage as an Adjunct Therapy for Crohn's Disease: a Randomized, Placebo‐controlled Study]. clinicaltrials.gov/ct2/show/NCT03467620 (accessed 17 October 2018).
Additional references
Asbridge 2012
Brant 1999
-
- Brant R, Sutherland L, Hilsden R. Examining the minimum important difference. Statistics in Medicine 1999;18(19):2593‐603. - PubMed
Coutts 1998
-
- Coutts AA, Pertwee RG. Evidence that cannabinoid‐induced inhibition of electrically evoked contractions of the myenteric plexus ‐ longitudinal muscle preparation of guinea‐pig small intestine can be modulated by Ca2+ and cAMP. Canadian Journal of Physiology and Pharmacology 1998;76:340–6. - PubMed
Egger 1997
Feagan 2004
-
- Feagan BG. 5‐ASA therapy for active Crohn's disease: old friends, old data, and a new conclusion. Clinical Gastroenterology and Hepatology 2004;2(5):376‐8. - PubMed
Fletcher 2013
Friedman 2012
-
- Friedman S, Blumberg RS. Chapter 295: Inflammatory Bowel Disease. Harrison’s Principles of Internal Medicine. 18th Edition. The McGraw‐Hill Companies, 2012.
Gubatan 2015
-
- Gubatan J, Staller K, Barshop K, Kuo B. Cannabis abuse is increasing and associated with increased emergency department utilization in gastroenterology patients. Digestive Diseases and Sciences 2016;61:1844–52. - PubMed
Guyatt 2008
Hackam 2015
-
- Hackam DG. Cannabis and stroke ‐ systematic appraisal of case reports. Stroke 2015;46:852‐6. - PubMed
Hasenoehrl 2016
Hauser 2014
Higgins 2011a
-
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
-
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviewsof Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hill 2015
-
- Hill KP. Medical marijuana for treatment of chronic pain and other medical and psychiatric problems: A clinical review. JAMA 2015;313(24):2474‐83. - PubMed
Irvine 1994
-
- Irvine EJ, Feagan B, Rochon J, Archambault A, Fedorak RN, Groll A, et al. Quality of life: a valid and reliable measure of therapeutic efficacy in the treatment of inflammatory bowel disease. Canadian Crohn's Relapse Prevention Trial Study Group. Gastroenterology 1994;106(2):287‐96. - PubMed
Irvine 2008
-
- Irvine EJ. Quality of life of patients with ulcerative colitis: past, present, and future. Inflammatory Bowel Diseases 2008;14(4):554‐65. - PubMed
Johnson 2005
-
- Johnson GJ, Cosnes J, Mansfield JC. Review article: smoking cessation as primary therapy to modify the course of Crohn’s disease. Alimentary Pharmacology and Therapeutics 2005;21:921–31. - PubMed
Klein 2006
-
- Klein TW, Carbral GA. Cannabinoid‐Induced immune suppression and modulation of antigen‐presenting cells. Journal of Neuroimmune Pharmacology 2006;1:50‐64. - PubMed
Lahat 2012
-
- Lahat A, Lang A, Ben‐Horin S. Impact of cannabis treatment on the quality of life, weight and clinical disease activity in Inflammatory Bowel Disease patients: A pilot prospective study. Digestion 2012;85:1‐8. - PubMed
Lal 2011
-
- Lal S, Prasad N, Ryan M, Tangri S, Silverberg MS, Gordon A, et al. Cannabis use amongst patients with inflammatory bowel disease. European Journal of Gastroenterology and Hepatology 2011;23(10):891‐6. - PubMed
Langhorst 2015
-
- Langhorst J, Wulfert H, Lauche R, Klose P, Cramer H, Dobos GJ, et al. Systematic review of complementary and alternative medicine treatments in inflammatory bowel diseases. Journal of Crohn's and Colitis 2015;9(1):86‐106. - PubMed
Marquez 2009
Massa 2004
Pinto 2002
-
- Pinto L, Izzo AA, Mascolo N, Capasso F, Cascio MG, Bisogno T, et al. Endocannabinoids as physiological regulators of colonic propulsion in mice. Gastroenterology 2002;123:227–34. - PubMed
Ramo 2012
Rubin 2004
-
- Rubin GP, Hungin AP, Chinn DJ, Dwarakanath D. Quality of life in patients with established inflammatory bowel disease: a UK general practice survey. Alimentary Pharmacology and Therapeutics 2004;19(5):529‐35. - PubMed
Schauer 2016
-
- Schauer GL, Berg CJ, Kegler MC, Donovan DM, Windle M. Differences in tobacco product use among past month adult marijuana users and nonusers: Findings from the 2003–2012 national survey on drug use and health. Nicotine and Tobacco Research 2016;18(3):281–8. - PubMed
Schauer 2017
-
- Schauer GL, Rosenberry ZR, Peters EN. Marijuana and tobacco co‐administration in blunts, spliffs, and mulled cigarettes: A systematic literature review. Addictive Behaviors 2017;64:200‐11. - PubMed
Schünemann 2011
-
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Singh 2012
Storr 2014
-
- Storr M, Devlin S, Kaplan GG, Panaccione R, Andrews CN. Cannabis use provides symptom relief in patients with inflammatory bowel disease but is associated with worse disease prognosis in patients with Crohn’s disease. Inflammatory Bowel Diseases 2014;20(3):472–80. - PubMed
Tibirica 2010
Triantafillidis 2013
-
- Triantafillidis JK, Merikas Em Gikas A. Psychological factors and stress in inflammatory bowel disease. Expert Review of Gastroenterology and Hepatology 2013;7(3):225‐38. - PubMed
Vianna 2012
Volz 2016
-
- Volz MS, Siegmund B, Hauser W. Efficacy, tolerability, and safety of cannabinoids in gastroenterology: A systematic review. Schmerz 2016;30(1):37‐46. - PubMed
Weiss 2015
-
- Weiss A, Friedenberg F. Patterns of cannabis use in patients with Inflammatory Bowel Disease: A population based analysis. Drug and Alcohol Dependence 2015;156:84‐9. - PubMed
Whiting 2015
-
- Whiting PF, Wolff RF, Deshpande S, Nisio M, Duffy S, Hernandez A, et al. Cannabinoids for medical use a systematic review and meta‐analysis. JAMA 2015;313(24):2456‐73. - PubMed
Wright 2005
-
- Wright K, Rooney N, Feeney M, Tate J, Robertson D, Welham M, et al. Differential expression of cannabinoid receptors in the human colon: Cannabinoids promote epithelial wound healing. Gastroenterology 2005;129(2):437‐53. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

