5 S,15 S-Dihydroperoxyeicosatetraenoic Acid (5,15-diHpETE) as a Lipoxin Intermediate: Reactivity and Kinetics with Human Leukocyte 5-Lipoxygenase, Platelet 12-Lipoxygenase, and Reticulocyte 15-Lipoxygenase-1
- PMID: 30407793
- PMCID: PMC7270142
- DOI: 10.1021/acs.biochem.8b00889
5 S,15 S-Dihydroperoxyeicosatetraenoic Acid (5,15-diHpETE) as a Lipoxin Intermediate: Reactivity and Kinetics with Human Leukocyte 5-Lipoxygenase, Platelet 12-Lipoxygenase, and Reticulocyte 15-Lipoxygenase-1
Abstract
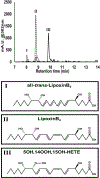
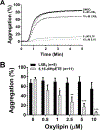
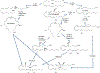
The reaction of 5 S,15 S-dihydroperoxyeicosatetraenoic acid (5,15-diHpETE) with human 5-lipoxygenase (LOX), human platelet 12-LOX, and human reticulocyte 15-LOX-1 was investigated to determine the reactivity and relative rates of producing lipoxins (LXs). 5-LOX does not react with 5,15-diHpETE, although it can produce LXA4 when 15-HpETE is the substrate. In contrast, both 12-LOX and 15-LOX-1 react with 5,15-diHpETE, forming specifically LXB4. For 12-LOX and 5,15-diHpETE, the kinetic parameters are kcat = 0.17 s-1 and kcat/ KM = 0.011 μM-1 s-1 [106- and 1600-fold lower than those for 12-LOX oxygenation of arachidonic acid (AA), respectively]. On the other hand, for 15-LOX-1 the equivalent parameters are kcat = 4.6 s-1 and kcat/ KM = 0.21 μM-1 s-1 (3-fold higher and similar to those for 12-HpETE formation by 15-LOX-1 from AA, respectively). This contrasts with the complete lack of reaction of 15-LOX-2 with 5,15-diHpETE [Green, A. R., et al. (2016) Biochemistry 55, 2832-2840]. Our data indicate that 12-LOX is markedly inferior to 15-LOX-1 in catalyzing the production of LXB4 from 5,15-diHpETE. Platelet aggregation was inhibited by the addition of 5,15-diHpETE, with an IC50 of 1.3 μM; however, LXB4 did not significantly inhibit collagen-mediated platelet activation up to 10 μM. In summary, LXB4 is the primary product of 12-LOX and 15-LOX-1 catalysis, if 5,15-diHpETE is the substrate, with 15-LOX-1 being 20-fold more efficient than 12-LOX. LXA4 is the primary product with 5-LOX but only if 15-HpETE is the substrate. Approximately equal proportions of LXA4 and LXB4 are produced by 12-LOX but only if LTA4 is the substrate, as described previously [Sheppard, K. A., et al. (1992) Biochim. Biophys. Acta 1133, 223-234].
Figures



Similar articles
-
Strict Regiospecificity of Human Epithelial 15-Lipoxygenase-2 Delineates Its Transcellular Synthesis Potential.Biochemistry. 2016 May 24;55(20):2832-40. doi: 10.1021/acs.biochem.5b01339. Epub 2016 May 13. Biochemistry. 2016. PMID: 27145229 Free PMC article.
-
Deciphering the Molecular Details of the Lipoxin Formation Mechanism in the 5(S),15(S)-DiHpETE Biosynthetic Pathway Catalyzed by Reticulocyte 15-Lipoxygenase-1.J Phys Chem B. 2020 Dec 17;124(50):11406-11418. doi: 10.1021/acs.jpcb.0c09147. Epub 2020 Dec 4. J Phys Chem B. 2020. PMID: 33274949
-
In Vitro Biosynthetic Pathway Investigations of Neuroprotectin D1 (NPD1) and Protectin DX (PDX) by Human 12-Lipoxygenase, 15-Lipoxygenase-1, and 15-Lipoxygenase-2.Biochemistry. 2021 Jun 8;60(22):1741-1754. doi: 10.1021/acs.biochem.0c00931. Epub 2021 May 24. Biochemistry. 2021. PMID: 34029049 Free PMC article.
-
The Role of 12/15-Lipoxygenase and Its Various Metabolites Generated from Multiple Polyunsaturated Fatty Acids as Substrates in Inflammatory Responses.Biomed Res Int. 2022 Sep 25;2022:4589191. doi: 10.1155/2022/4589191. eCollection 2022. Biomed Res Int. 2022. Retraction in: Biomed Res Int. 2023 Jun 21;2023:9810176. doi: 10.1155/2023/9810176. PMID: 36199753 Free PMC article. Retracted. Review.
-
The two faces of the 15-lipoxygenase in atherosclerosis.Prostaglandins Leukot Essent Fatty Acids. 2007 Aug;77(2):67-77. doi: 10.1016/j.plefa.2007.08.001. Epub 2007 Sep 14. Prostaglandins Leukot Essent Fatty Acids. 2007. PMID: 17869078 Review.
Cited by
-
Research progress on the role of lipoxygenase and its inhibitors in prostate cancer.Future Oncol. 2024 Dec;20(40):3549-3568. doi: 10.1080/14796694.2024.2419356. Epub 2024 Nov 13. Future Oncol. 2024. PMID: 39535136 Review.
-
Role of Human 15-Lipoxygenase-2 in the Biosynthesis of the Lipoxin Intermediate, 5S,15S-diHpETE, Implicated with the Altered Positional Specificity of Human 15-Lipoxygenase-1.Biochemistry. 2020 Oct 27;59(42):4118-4130. doi: 10.1021/acs.biochem.0c00622. Epub 2020 Oct 13. Biochemistry. 2020. PMID: 33048542 Free PMC article.
-
The Role of Hydroxyeicosatetraenoic Acids in the Regulation of Inflammation in Bronchial Asthma.Dokl Biochem Biophys. 2024 Dec;519(1):512-520. doi: 10.1134/S1607672924701126. Epub 2024 Sep 16. Dokl Biochem Biophys. 2024. PMID: 39283556 Review.
-
Metabolism pathways of arachidonic acids: mechanisms and potential therapeutic targets.Signal Transduct Target Ther. 2021 Feb 26;6(1):94. doi: 10.1038/s41392-020-00443-w. Signal Transduct Target Ther. 2021. PMID: 33637672 Free PMC article. Review.
-
15-Lipoxygenase-1 biosynthesis of 7S,14S-diHDHA implicates 15-lipoxygenase-2 in biosynthesis of resolvin D5.J Lipid Res. 2020 Jul;61(7):1087-1103. doi: 10.1194/jlr.RA120000777. Epub 2020 May 13. J Lipid Res. 2020. PMID: 32404334 Free PMC article.
References
-
- Serhan CN, Hamberg M, and Samuelsson B (1984) Trihydroxytetraenes: a novel series of compounds formed from arachidonic acid in human leukocytes, Biochem Biophys Res Commun 118, 943–949. - PubMed
-
- Romano M, Cianci E, Simiele F, and Recchiuti A (2015) Lipoxins and aspirin-triggered lipoxins in resolution of inflammation, Eur J Pharmacol 760, 49–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

