Long-Term Survival of Patients With Melanoma With Active Brain Metastases Treated With Pembrolizumab on a Phase II Trial
- PMID: 30407895
- PMCID: PMC6354772
- DOI: 10.1200/JCO.18.00204
Long-Term Survival of Patients With Melanoma With Active Brain Metastases Treated With Pembrolizumab on a Phase II Trial
Abstract
Purpose: Pembrolizumab is active in melanoma, but activity in patients with untreated brain metastasis is less established. We present long-term follow-up of pembrolizumab-treated patients with new or progressing brain metastases treated on a phase II clinical trial ( ClinicalTrials.gov identifier: NCT02085070).
Patients and methods: We enrolled 23 patients with melanoma with one or more asymptomatic, untreated 5- to 20-mm brain metastasis not requiring corticosteroids; 70% of patients had prior systemic therapy. Pembrolizumab was administered for up to 24 months. Brain metastasis response, the primary end point, was assessed by modified Response Evaluation Criteria in Solid Tumors (RECIST). Pretreatment tumors were analyzed for T-cell infiltrate and programmed death ligand 1.
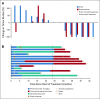
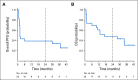
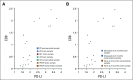
Results: Six patients (26%) had a brain metastasis response. Eight patients (35%) did not reach a protocol evaluation scan and were unevaluable for brain metastasis response as a result of progression or need for radiation. Brain metastasis and systemic responses were concordant, with all ongoing at 24 months. The median progression-free and overall survival times were 2 and 17 months, respectively. Eleven patients (48%) were alive at 24 months. This included three unevaluable patients. One of these three patients had hemorrhaged, and two had symptoms from perilesional edema requiring radiosurgery, but all three patients remained on commercial pembrolizumab more than 24 months later. None of the 24-month survivors received subsequent BRAF inhibitors. Neurologic adverse events occurred in 65% of patients; all adverse events but one were grade 1 or 2. Three patients had seizures, which were treated with anticonvulsants. Most responders had higher pretreatment tumor CD8 cell density and programmed death ligand 1 expression, whereas all nonresponders did not.
Conclusion: Pembrolizumab is active in melanoma brain metastases with acceptable toxicity and durable responses. Multidisciplinary care is required to optimally manage patients with brain metastases, including consideration of radiation to large or symptomatic lesions, which were excluded in this trial. Two-year survival was similar to patients without brain metastasis treated with anti-programmed cell death 1 agents. Concordant brain and extracerebral responses support use of pembrolizumab to treat small, asymptomatic brain metastases.
Figures

Comment in
-
Can Immunotherapy Replace Radiotherapy in Melanoma Brain Metastases?J Clin Oncol. 2019 Apr 20;37(12):1030-1031. doi: 10.1200/JCO.18.01982. Epub 2019 Feb 26. J Clin Oncol. 2019. PMID: 30807232 No abstract available.
-
Reply to A. Shinde et al.J Clin Oncol. 2019 Apr 20;37(12):1031-1032. doi: 10.1200/JCO.18.02463. Epub 2019 Feb 26. J Clin Oncol. 2019. PMID: 30807233 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

