Harnessing olfactory bulb oscillations to perform fully brain-based sleep-scoring and real-time monitoring of anaesthesia depth
- PMID: 30408025
- PMCID: PMC6224033
- DOI: 10.1371/journal.pbio.2005458
Harnessing olfactory bulb oscillations to perform fully brain-based sleep-scoring and real-time monitoring of anaesthesia depth
Abstract
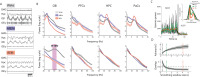
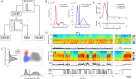
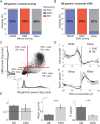
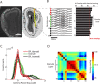
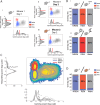
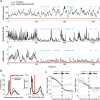
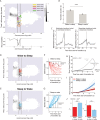
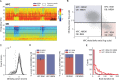
Real-time tracking of vigilance states related to both sleep or anaesthesia has been a goal for over a century. However, sleep scoring cannot currently be performed with brain signals alone, despite the deep neuromodulatory transformations that accompany sleep state changes. Therefore, at heart, the operational distinction between sleep and wake is that of immobility and movement, despite numerous situations in which this one-to-one mapping fails. Here we demonstrate, using local field potential (LFP) recordings in freely moving mice, that gamma (50-70 Hz) power in the olfactory bulb (OB) allows for clear classification of sleep and wake, thus providing a brain-based criterion to distinguish these two vigilance states without relying on motor activity. Coupled with hippocampal theta activity, it allows the elaboration of a sleep scoring algorithm that relies on brain activity alone. This method reaches over 90% homology with classical methods based on muscular activity (electromyography [EMG]) and video tracking. Moreover, contrary to EMG, OB gamma power allows correct discrimination between sleep and immobility in ambiguous situations such as fear-related freezing. We use the instantaneous power of hippocampal theta oscillation and OB gamma oscillation to construct a 2D phase space that is highly robust throughout time, across individual mice and mouse strains, and under classical drug treatment. Dynamic analysis of trajectories within this space yields a novel characterisation of sleep/wake transitions: whereas waking up is a fast and direct transition that can be modelled by a ballistic trajectory, falling asleep is best described as a stochastic and gradual state change. Finally, we demonstrate that OB oscillations also allow us to track other vigilance states. Non-REM (NREM) and rapid eye movement (REM) sleep can be distinguished with high accuracy based on beta (10-15 Hz) power. More importantly, we show that depth of anaesthesia can be tracked in real time using OB gamma power. Indeed, the gamma power predicts and anticipates the motor response to stimulation both in the steady state under constant anaesthetic and dynamically during the recovery period. Altogether, this methodology opens the avenue for multi-timescale characterisation of brain states and provides an unprecedented window onto levels of vigilance.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Nasal Respiration is Necessary for the Generation of γ Oscillation in the Olfactory Bulb.Neuroscience. 2019 Feb 1;398:218-230. doi: 10.1016/j.neuroscience.2018.12.011. Epub 2018 Dec 13. Neuroscience. 2019. PMID: 30553790
-
Design and validation of a computer-based sleep-scoring algorithm.J Neurosci Methods. 2004 Feb 15;133(1-2):71-80. doi: 10.1016/j.jneumeth.2003.09.025. J Neurosci Methods. 2004. PMID: 14757347
-
Scoring transitions to REM sleep in rats based on the EEG phenomena of pre-REM sleep: an improved analysis of sleep structure.Sleep. 1994 Feb;17(1):28-36. doi: 10.1093/sleep/17.1.28. Sleep. 1994. PMID: 8191200
-
State transitions between wake and sleep, and within the ultradian cycle, with focus on the link to neuronal activity.Sleep Med Rev. 2004 Dec;8(6):473-85. doi: 10.1016/j.smrv.2004.06.006. Sleep Med Rev. 2004. PMID: 15556379 Review.
-
Basic sleep mechanisms: an integrative review.Cent Nerv Syst Agents Med Chem. 2012 Mar;12(1):38-54. doi: 10.2174/187152412800229107. Cent Nerv Syst Agents Med Chem. 2012. PMID: 22524274 Review.
Cited by
-
Pharmacological inhibition of S6K1 rescues synaptic deficits and attenuates seizures and depression in chronic epileptic rats.CNS Neurosci Ther. 2024 Mar;30(3):e14475. doi: 10.1111/cns.14475. Epub 2023 Sep 22. CNS Neurosci Ther. 2024. PMID: 37736829 Free PMC article.
-
Reciprocal relationships between sleep and smell.Front Neural Circuits. 2022 Dec 22;16:1076354. doi: 10.3389/fncir.2022.1076354. eCollection 2022. Front Neural Circuits. 2022. PMID: 36619661 Free PMC article. Review.
-
Understanding How Acute Alcohol Impacts Neural Encoding in the Rodent Brain.Curr Top Behav Neurosci. 2024 Jun 11:10.1007/7854_2024_479. doi: 10.1007/7854_2024_479. Online ahead of print. Curr Top Behav Neurosci. 2024. PMID: 38858298 Free PMC article.
-
Decoding behavior from global cerebrovascular activity using neural networks.Sci Rep. 2023 Mar 2;13(1):3541. doi: 10.1038/s41598-023-30661-5. Sci Rep. 2023. PMID: 36864293 Free PMC article.
-
Enhancement of Motor Cortical Gamma Oscillations and Sniffing Activity by Medial Forebrain Bundle Stimulation Precedes Locomotion.eNeuro. 2022 Jul 19;9(4):ENEURO.0521-21.2022. doi: 10.1523/ENEURO.0521-21.2022. Print 2022 Jul-Aug. eNeuro. 2022. PMID: 35701167 Free PMC article.
References
-
- Steriade M, McCormick D, Sejnowski T. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262(5134):679–85. - PubMed
-
- Cirelli C, Tononi G. Gene expression in the brain across the sleep–waking cycle. Brain Res. 2000. December;885(2):303–21. - PubMed
-
- Benington JH, Heller HC. Restoration of brain energy metabolism as the function of sleep. Prog Neurobiol. 1995. March;45(4):347–60. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

