Substrate-independent immunomodulatory characteristics of mesenchymal stem cells in three-dimensional culture
- PMID: 30408051
- PMCID: PMC6224081
- DOI: 10.1371/journal.pone.0206811
Substrate-independent immunomodulatory characteristics of mesenchymal stem cells in three-dimensional culture
Abstract
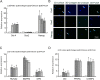
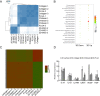
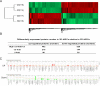
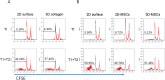
Mesenchymal stem cells (MSCs) play important roles in tissue regeneration, and multi-lineage differentiation and immunomodulation are two major characteristics of MSCs that are utilized in stem cell therapy. MSCs in vivo have a markedly different three-dimensional (3D) niche compared to the traditional two-dimensional (2D) culture in vitro. A 3D scaffold is predicted to provide an artificial 3D environment similar to the in vivo environment. Significant changes in MSC differentiation are shown to be occurred when under 3D culture. However, the immunomodulatory characteristics of MSCs under 3D culture remain unknown. In this study, 3D culture systems were constructed using different substrates to evaluate the common immunomodulatory characteristics of MSCs. Compared to the MSCs under 2D culture, the MSCs under 3D culture, which had higher stemness and maintained cell phenotype, showed altered immunophenotypic pattern. Gene expression profile analysis at mRNA and protein level detected by gene chip and protein chip, respectively, further revealed the difference between 3D cultured MSCs and 2D cultured MSCs, which was mainly concentrated in the immunoregulation related aspects. Moreover, the immunoregulatory role of 3D culture was confirmed by our immunosuppressive experiments. These findings demonstrated that the immunomodulatory capacities of MSCs were enhanced by the 3D geometry of substrates.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Changes in the secretome of tri-dimensional spheroid-cultured human mesenchymal stem cells in vitro by interleukin-1 priming.Stem Cell Res Ther. 2018 Jan 17;9(1):11. doi: 10.1186/s13287-017-0753-5. Stem Cell Res Ther. 2018. PMID: 29343288 Free PMC article.
-
The three-dimensional collagen scaffold improves the stemness of rat bone marrow mesenchymal stem cells.J Genet Genomics. 2012 Dec 20;39(12):633-41. doi: 10.1016/j.jgg.2012.08.006. Epub 2012 Nov 21. J Genet Genomics. 2012. PMID: 23273767
-
3D Decellularized Native Extracellular Matrix Scaffold for In Vitro Culture Expansion of Human Wharton's Jelly-Derived Mesenchymal Stem Cells (hWJ MSCs).Methods Mol Biol. 2018;1577:35-53. doi: 10.1007/7651_2017_71. Methods Mol Biol. 2018. PMID: 28963712
-
Increased Paracrine Immunomodulatory Potential of Mesenchymal Stromal Cells in Three-Dimensional Culture.Tissue Eng Part B Rev. 2016 Aug;22(4):322-9. doi: 10.1089/ten.TEB.2015.0532. Epub 2016 Mar 16. Tissue Eng Part B Rev. 2016. PMID: 26861485 Free PMC article. Review.
-
3D Bone Biomimetic Scaffolds for Basic and Translational Studies with Mesenchymal Stem Cells.Int J Mol Sci. 2018 Oct 13;19(10):3150. doi: 10.3390/ijms19103150. Int J Mol Sci. 2018. PMID: 30322134 Free PMC article. Review.
Cited by
-
Cryo-Temperature Pretreatment Increases the Pro-Angiogenic Capacity of Three-Dimensional Mesenchymal Stem Cells via the PI3K-AKT Pathway.Cell Transplant. 2022 Jan-Dec;31:9636897221106996. doi: 10.1177/09636897221106996. Cell Transplant. 2022. PMID: 35727010 Free PMC article.
-
Murine bone-derived mesenchymal stem cells undergo molecular changes after a single passage in culture.Sci Rep. 2024 May 29;14(1):12396. doi: 10.1038/s41598-024-63009-8. Sci Rep. 2024. PMID: 38811646 Free PMC article.
-
Strategies to Potentiate Paracrine Therapeutic Efficacy of Mesenchymal Stem Cells in Inflammatory Diseases.Int J Mol Sci. 2021 Mar 25;22(7):3397. doi: 10.3390/ijms22073397. Int J Mol Sci. 2021. PMID: 33806241 Free PMC article. Review.
-
3D Scaffolds to Model the Hematopoietic Stem Cell Niche: Applications and Perspectives.Materials (Basel). 2021 Jan 26;14(3):569. doi: 10.3390/ma14030569. Materials (Basel). 2021. PMID: 33530372 Free PMC article. Review.
-
Bone Marrow Niches of Hematopoietic Stem and Progenitor Cells.Int J Mol Sci. 2022 Apr 18;23(8):4462. doi: 10.3390/ijms23084462. Int J Mol Sci. 2022. PMID: 35457280 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

